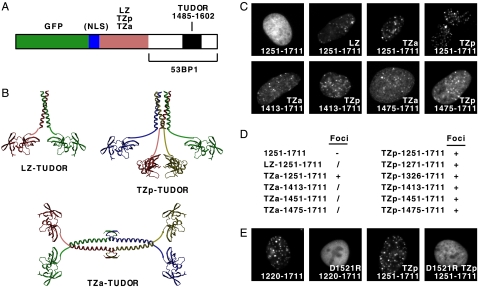
FIG. 3.
Heterologous oligomerization domains can restore the focus-forming activity of GFP-53BP1 fusion proteins lacking the endogenous 53BP1 oligomerization domain. (A) Diagram of the GFP-53BP1 fusion proteins containing a heterologous oligomerization domain between the GFP and 53BP1 sequences and, optionally, also an NLS. LZ, leucine zipper; TZp, modified tetrameric leucine zipper with parallel α-helices; TZa, tetramerization domain generated by fusing part of the p53 tetramerization domain to a leucine zipper, resulting in two coiled coils packing antiparallel to each other. (B) Ribbon representations of the three-dimensional structures of segments of the 53BP1 fusion proteins corresponding to the heterologous oligomerization domain and the tandem tudor domain of 53BP1. The images were generated using the programs MOLSCRIPT and RASTER3D from Protein Data Bank files: 2ZTA, GCN4 leucine zipper (LZ); 1GCL, modified tetrameric GCN4 leucine zipper with parallel α-helices (TZp); 1C26 and 2ZTA, human p53 tetramerization domain and GCN4 leucine zipper (TZa); and 1XNI, tandem tudor domain of human 53BP1 (TUDOR). (C to E) Focus-forming activities of GFP-53BP1 fusion proteins containing various segments of human 53BP1 and, optionally, a heterologous oligomerization domain and/or a D1521R substitution in the 53BP1 tudor domain, as indicated. Focus-forming activities were classified as described in the legend to Fig. 1.