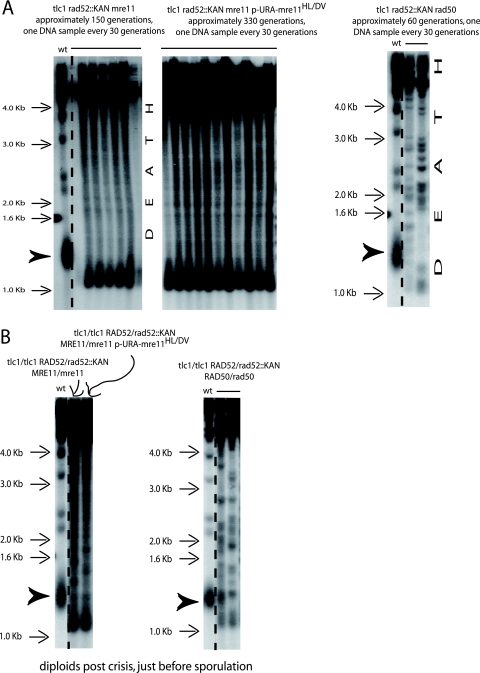
FIG. 5.
Mre11 and Rad50 are essential to the ILT pathway, but Mre11 nuclease activity is dispensable for this process. (A) Left panel, deletion of MRE11 leads to the death of tlc1Δ rad52Δ cells which have undergone the senescence process with overelongated telomeres (see Fig. 1B, left panel). Middle panel, telomere organization in ILT survivors from tlc1Δ rad52Δ mre11Δ cells harboring the nuclease-deficient mre11-H125L-D126V allele on a centromeric plasmid (recombining in type II-ALT at the onset of senescence, at the beginning of the kinetics shown here). Right panel, the tlc1Δ rad52Δ rad50Δ cells that had undergone senescence in the presence of overelongated telomeres also died, but earlier than the mre11Δ mutant. The arrowheads indicate the size of the bulk of wild-type telomeres Three, four, and six experiments were performed in total for the strains illustrated in the left, middle, and right panels, respectively. (B) Since the pattern of telomere organization in the mre11-H125L-D126V ILT survivors was different from that in the other ILT survivors (see, for instance, Fig. 2A and 4A and B) and the pattern in mre11Δ cells was different from that in rad50Δ cells, as seen above, telomere organization in the parental diploid strains was analyzed. The mre11 pattern (darkened here, as well as in panel A, to visualize intermediate-size bands), characterized by a more abundant species of the smaller bands, was also present in the parental diploid strain (left panel), while the rad50 diploid exhibited a normal type II-like pattern (right panel). We assume that haploinsufficiency of MRE11 is probably the cause for this observed atypical type II pattern, with the smaller bands accounting for a tendency to type I recombination due to limiting amounts of Mre11 in the diploid (Mre11 is essential for type II-ALT recombination). A TG1-3 32P-labeled probe was used with XhoI cutting.