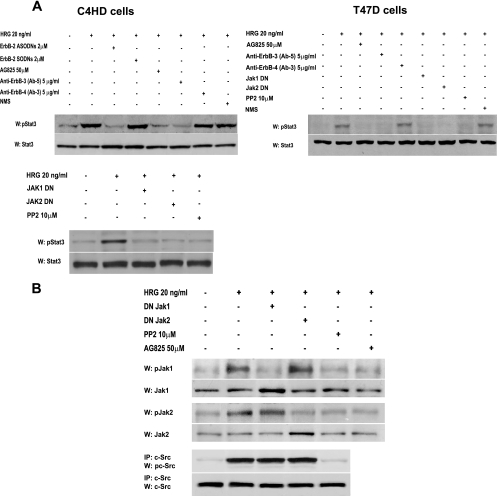
FIG. 2.
Participation of ErbBs, Jaks, and c-Src in HRG-induced Stat3 phosphorylation. (A) C4HD (left) and T47D (right) cells were preincubated for 90 min with AG825, with monoclonal antibodies to either ErbB-3 or ErbB-4, and with normal mouse serum (NMS), transiently transfected with 2 μg of DN Jak1 or DN Jak2 vector for 2 days, and preincubated with PP2 for 90 min. C4HD cells were also pretreated for 48 h with either ASODNs or SODNs to ErbB-2. Cells were then treated with HRG for 10 min or left untreated. Fifty micrograms of protein from C4HD or T47D cell lysate was electrophoresed, and Western blots were performed with phosphotyrosine 705 Stat3 antibody. Membranes were stripped and hybridized with total Stat3 antibody. Phospho-Stat3 bands underwent densitometry, and values were normalized to total protein bands. Data analysis showed that the increase in Stat3 phosphorylation in cells treated with HRG compared with that in untreated cells and the inhibition of HRG-induced Stat3 phosphorylation levels by ErbB-2 ASODNs, AG825, ErbB-3 antibody, DN Jaks, and PP2 were significant (P < 0.001). These experiments were repeated five times with similar results. (B) Interplay among ErbB-2, Jaks, and Src. C4HD cells were preincubated with PP2 and AG825 or transiently transfected with 2 μg of DN Jak1 or DN Jak2 vector, as described for panel A, before treatment with HRG. Fifty micrograms of protein from cell lysates was immunoblotted with phosphotyrosine Jak1 and Jak2 antibodies. Membranes were stripped and hybridized with total Jak antibodies. c-Src activity in cell lysates was determined as described in the legend to Fig. 1. Phospho- and total Jak and c-Src bands underwent densitometry and were analyzed as detailed in the legend to Fig. 1, showing significant (P< 0.001) induction of Jak and c-Src phosphorylation by HRG and significant (P < 0.001) inhibition of HRG-induced Jak phosphorylation by PP2 and AG825. This experiment was repeated three times with similar results.