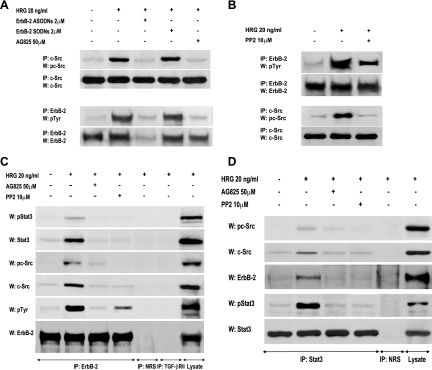
FIG. 3.
HRG-induced interactions among ErbB-2, c-Src, and Stat3. (A and B) Interplay between ErbB-2 and c-Src in C4HD cells. (A) ErbB-2 expression and activation were blocked using ASODNs and AG825 respectively, as described in the legend to Fig. 2, and cells were treated with HRG for 10 min or left untreated. c-Src activity in cell lysates was determined as described in the legend to Fig. 1. ErbB-2 was immunoprecipitated from 250 μg protein with an ErbB-2 antibody, and the blot was revealed with a phosphotyrosine (p-Tyr) antibody. Identical aliquots of each immunoprecipitate were subjected to immunoblot analysis with the ErbB-2 antibody to verify that nearly equal amounts of immunoprecipitated proteins were loaded. Phospho-c-Src bands underwent densitometry, and values were normalized to total protein bands, showing that the increase in c-Src phosphorylation levels by HRG compared with those in untreated cells and the inhibition of HRG-induced c-Src phosphorylation by ErbB-2 ASODNs or AG825 were significant (P < 0.001). (B) Protein extracts from cells preincubated with PP2 before HRG treatment were analyzed for ErbB-2 and c-Src activation as described for panel A Phosphotyrosine bands in ErbB-2-immunoprecipitated extracts underwent densitometry and were normalized to total ErbB-2 bands, showing a significant (P< 0.001) increase in ErbB-2 phosphorylation levels by HRG and significant (P < 0.001) inhibition of HRG-induced ErbB-2 phosphorylation by PP2. The experiments shown in panels A and B were repeated three times with similar results. (C and D) Association among ErbB-2, c-Src, and Stat3. (C) Protein extracts (1 mg) from C4HD cells preincubated with AG825 or PP2 before HRG stimulation were immunoprecipitated with an ErbB-2 antibody and analyzed by Western blotting with phosphotyrosine 705 Stat3, phosphotyrosine 418/423 c-Src, total phosphotyrosine, and ErbB-2 antibodies. Membranes were then stripped and revealed with total Stat3, c-Src, and ErbB-2 antibodies. As controls for the specificity of the protein interactions, lysates were also immunoprecipitated with NRS and with TGF-βRII antibody. Cell lysates were blotted in parallel using the indicated antibodies. (D) C4HD protein extracts (1 mg) treated as described for panel C were immunoprecipitated with a Stat3 antibody and analyzed by Western blotting with phosphotyrosine 418/423 c-Src, ErbB-2, and phosphotyrosine 705 Stat3 antibodies. Membranes were then stripped and revealed with total c-Src and Stat3 antibodies. As a control, lysates were also immunoprecipitated with NRS. Cell lysates were blotted in parallel, using the indicated antibodies. The experiments shown in panels C and D were repeated three times with similar results.