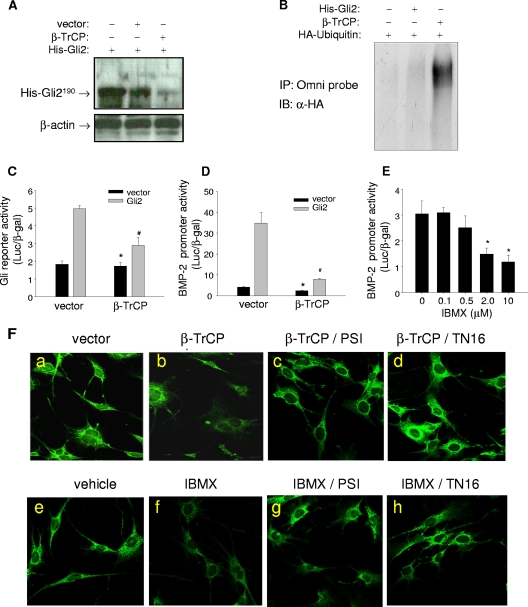
FIG. 6.
Effects of inhibition of microtubule assembly on β-TrCP-mediated Gli2 degradation. (A) Western blot of Gli2. C3H10T1/2 cells were cotransfected with His-Gli2 and β-TrCP expression plasmids, and after 24 h, cells were lysed and proteins were separated by SDS-PAGE and probed with a rabbit Omni-probe anti-His antibody. β-Actin is the loading control. (B) Ubiquitination of Gli2. C3H10T1/2 cells were cotransfected with His-Gli2, HA-ubiquitin, and β-TrCP plasmids. Cell lysates were incubated with an Omni-probe anti-His antibody and protein A beads. The immunoprecipitate (IP) was separated by SDS-PAGE and blotted (immunoblot [IB]) with anti-HA (α-HA) antibody. (C and D) Effects of β-TrCP on luciferase reporter activity. 2T3 cells were cotransfected with Gli2, β-TrCP plasmids, and a Gli response reporter construct, 8×3′Gli-BSδ5/LucII (C) or the BMP-2 promoter reporter, −2712/+165-Luc (D). Luciferase activity was measured with β-Gal normalization. (E) Effects of IBMX on BMP-2 promoter activity. The BMP-2-Luc 2T3 cells were incubated with IBMX at doses from 0.1 to 10 μM for 48 h. The reporter luciferase activity was determined. (F) Immunofluorescence of Gli2. 2T3 cells seeded in eight-well chamber slides with different treatments were processed for immunofluorescence assay using anti-mouse Gli2 antibody and FITC-labeled secondary antibody. Controls (Ea and e), β-TrCP transfection (Eb), or IBMX (2 μM) incubation (Ef), β-TrCP with 100 nM PSI (Ec), β-TrCP with 1 μM TN16 (Ed), IBMX with PSI (Eg), or IBMX with TN16 (Eh) is shown. *, P < 0.05 versus results with vector (black); #, P < 0.05 versus results with vector (gray).