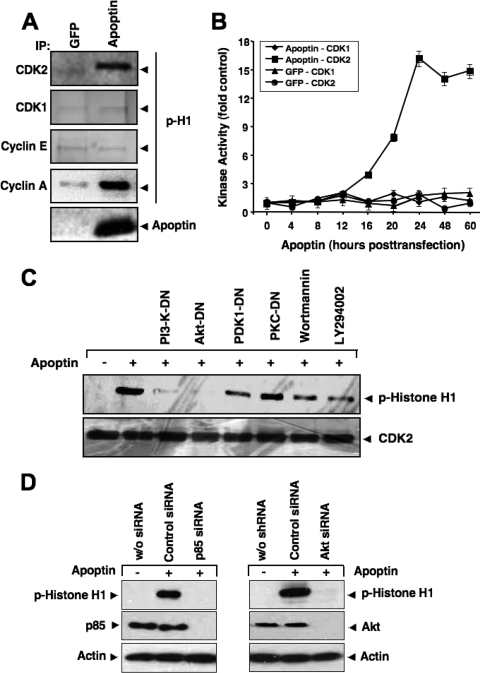
FIG. 3.
CDK2 is activated during apoptin-induced cell death. (A) CDK2, CDK1, cyclin E, and cyclin A were immunoprecipitated from MCF-7 cells transfected with either GFP or GFP-apoptin at 24 h posttransfection and used in an in vitro kinase assay with histone H1 as the substrate. The level of histone H1 phosphorylation was detected by immunoblotting with a phospho-specific antibody against histone H1. (B) The kinase activities of CDK1 and CDK2 at different times after transfection with either GFP or apoptin were measured and plotted. Immunoblot signals were quantified against the respective controls, using a Storm scanner and accompanying software. (C) PC3 cells were transfected with apoptin alone or together with different dominant-negative mutants of PI3-K, Akt, PDK1, or PKCɛ. Kinase activity of immunoprecipitated CDK2 was measured using histone H1 as the substrate 24 h after transfection. Total CDK2 levels were determined by Western blotting. The PI3-K inhibitors wortmannin (5 nM) and LY294002 (1.5 μM) were applied 1 h before cell lysis. (D) Knockdown of PI3-K and Akt abolishes apoptin-induced CDK2 activation. PC3 cells were transfected with either control siRNA, a PI3-K p85-specific siRNA (left), or an Akt-specific siRNA (right). RNA interference resulted in an almost complete knockdown of p85 and Akt expression. At 72 h posttransfection, cells were treated with Tat-apoptin or left untreated. The kinase activity of immunoprecipitated CDK2 was determined by using histone H1 as the substrate 24 h after apoptin treatment. The level of histone H1 phosphorylation was detected by immunoblotting with a phospho-specific antibody against histone H1. Actin served as a loading control.