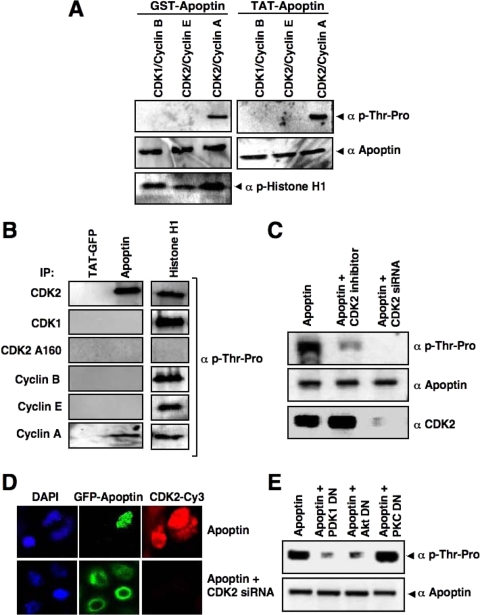
FIG. 5.
CDK2 is the specific apoptin kinase. (A) A nonradioactive in vitro kinase assay was performed with recombinant GST-apoptin and Tat-apoptin as substrates, using active CDK1-cyclin B, CDK2-cyclin E, or CDK2-cyclin A. Apoptin phosphorylation was detected by immunoblotting using an antibody against phosphorylated threonine-proline residues. Total apoptin levels were detected by apoptin antibody. Histone H1 was used as a positive control. (B) Active CDK2, CDK1, cyclin B, cyclin E, and cyclin A were immunoprecipitated from PC3 cells with their respective antibodies and the CDK2 T160A mutant was immunoprecipitated with anti-hemagglutinin antibody, and the immunoprecipitates were used in a kinase assay with Tat-GFP, Tat-apoptin, or histone H1 as the substrate. Phosphorylation was detected as described in the legend to Fig. 4A. (C) PC3 cells were transfected with GFP-apoptin alone or in the presence of the CDK2 inhibitor or the CDK2-targeting siRNA. GFP-apoptin was immunoprecipitated at 24 h posttransfection with anti-GFP antibodies. The phosphorylation of apoptin in the immunoprecipitates was detected by immunoblotting using anti-phospho-Thr-Pro antibodies. Total apoptin and CDK2 levels are indicated. (D) The effect of CDK2 inhibition on apoptin's localization was demonstrated in PC3 cells transfected with GFP-apoptin in the absence or presence of a CDK2-targeting siRNA plasmid. After 24 h, cells were analyzed by confocal laser scanning microscopy. (E) PI3-K/Akt signaling is required for apoptin phosphorylation. PC3 cells were transfected with GFP-apoptin in the presence or absence of a dominant-negative mutant of PDK1, Akt, or PKCɛ. The levels of apoptin phosphorylation were determined at 24 h posttransfection as described for panel C. Total apoptin levels were detected with anti-apoptin antibodies.