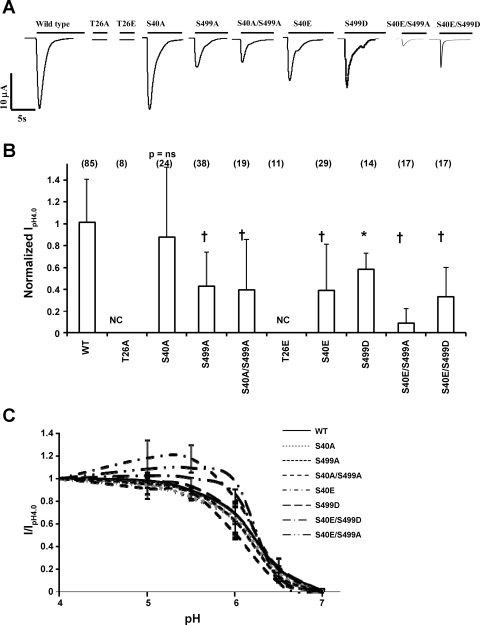
Fig. 2.
Mutations in PKC consensus phosphorylation sites on hASIC1b affect its function in Xenopus oocytes. cRNA (11.5 ng) for wild type (WT) hASIC1b or hASIC1b PKC phosphorylation mutants was expressed in Xenopus oocytes. Acid-activated currents at pH 4.0 were measured by two-electrode voltage clamp (TEV) at 1–4 days postinjection as described in materials and methods. A: representative traces of acid-activated currents of WT hASIC1b and each hASIC1b mutant. B: peak pH 4.0 current (IpH4.0) for each phosphorylation mutant was normalized to the average peak IpH4.0 recorded on the same day in oocytes of the same batch expressing WT hASIC1b. Shown is the summary of 3–6 experiments; values are means + SD. The number of individual oocytes recorded is shown in parentheses on top of each bar. P values were determined with one-way ANOVA with Tukey's post-hoc test. *P < 0.01 and †P < 0.001 vs. WT. NC, no acid-activated current. C: pH activation curves for WT hASIC1b and hASIC1b phosphorylation mutants. Peak acid-activated currents were recorded by switching the extracellular solution to pH 7.0, 6.5, 6.0, 5.5, 5.0, and 4.0 sequentially, as described in materials and methods, and were normalized to peak IpH4.0. Normalized values (I/IpH4.0) were fitted to the Hill equation to obtain pH50 values and Hill coefficients. n = 13 oocytes for WT, 10 oocytes for S40A, 11 oocytes for S499A, 8 oocytes for S40A/S499A, 8 oocytes for S40E, 8 oocytes for S499D, 5 oocytes for S40E/S499A, and 5 oocytes for S40E/S499D. T26A (n = 8) and T26E (n = 8) constructs did not exhibit any acid-activated current at any pH (data not shown).