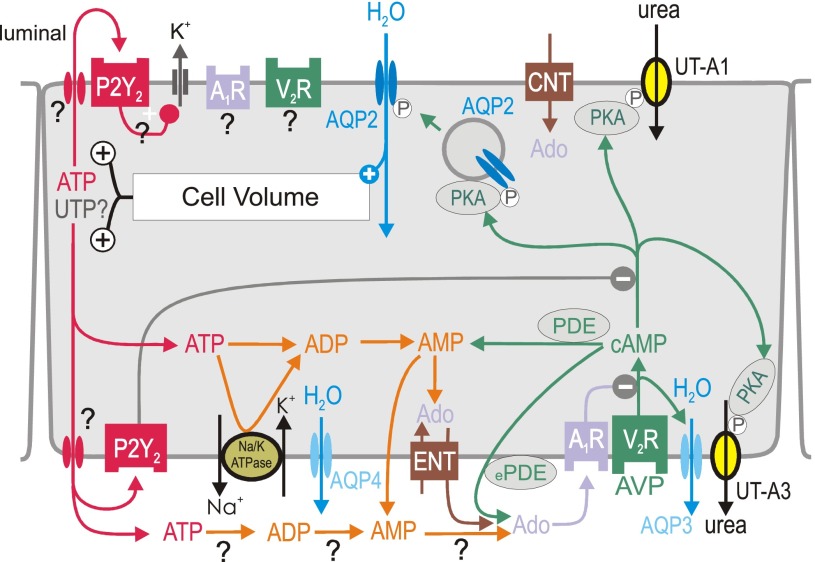
Fig. 4.
Proposed integrated model for local water transport inhibition by activation of P2Y2 and adenosine A1 receptors (A1R) in inner medullary collecting duct principal cell. Activation of vasopressin V2 receptors (V2R) by arginine-vasopressin (AVP) stimulates aquaporin-2 (AQP2)-mediated water flux, which increases cell volume. The latter increases basolateral and apical release of ATP (and potentially UTP?) by unknown mechanism. Basolateral water exit is mediated by constitutively expressed aquaporin-4 (AQP4) and AVP-regulated aquaporin-3 (AQP3) water channels. Urea transporters UT-A1 and UT-A3 play key roles in the urinary concentrating mechanism and fluid homeostasis; both are regulated by AVP. P2Y2 receptor activation may inhibit water reabsorption by (I) stimulation of volume-regulatory K+ channels, and (II) inhibition of protein kinase A (PKA). ATP may also be broken down to adenosine (Ado). Alternative sources of extracellular Ado are the extracellular cAMP-Ado pathway (conversion by extracellular phosphodiesterase, ePDE, and ecto-5′-nucleotidase) or transport mediated by equilibrative nucleoside transporter (ENT). To which extent extracellular ATP is degraded by ectonucleotidases to ADP, AMP, and Ado at the luminal (not shown) and basolateral surface is unclear. Activation of A1R and P2Y2 receptors inhibits AVP-stimulated water flux, which helps to normalize cell volume and accelerates the excretion of free water in response to water loading before circulating AVP levels fall. AC, adenylyl cyclase; AQP-3, -4, aquaporin-3, -4; CNT, concentrative nucleoside transporter; COX-1, cyclooxygenase 1; DAG, diacylglycerol; P, phosphorylation. See also text for details.