Abstract
Pre–B-cell leukemia spontaneously develops in BLNK-deficient mice, and pre–B-cell acute lymphoblastic leukemia cells in children often lack BLNK protein expression, demonstrating that BLNK functions as a tumor suppressor. However, the mechanism by which BLNK suppresses pre–B-cell leukemia, as well as the identification of other genetic alterations that collaborate with BLNK deficiency to cause leukemogenesis, are still unknown. Here, we demonstrate that the JAK3/STAT5 signaling pathway is constitutively activated in pre-B leukemia cells derived from BLNK−/− mice, mostly due to autocrine production of IL-7. Inhibition of IL-7R signaling or JAK3/STAT5 activity resulted in the induction of p27kip1 expression and cell-cycle arrest, accompanied by apoptosis in the leukemia cells. Transgene-derived constitutively active STAT5 (STAT5b-CA) strongly synergized with the loss of BLNK to initiate leukemia in vivo. In the leukemia cells, exogenously expressed BLNK inhibited autocrine JAK3/STAT5 signaling, resulting in p27kip1 induction, cell-cycle arrest, and apoptosis. BLNK-inhibition of JAK3 was dependent on the binding of BLNK to JAK3. These data indicate that BLNK normally regulates IL-7–dependent proliferation and survival of pre–B cells through direct inhibition of JAK3. Thus, somatic loss of BLNK and concomitant mutations leading to constitutive activation of Jak/STAT5 pathway result in the generation of pre–B-cell leukemia.
Introduction
In early B-cell development, successful rearrangement of the immunoglobulin (Ig) heavy (H) chain gene in progenitor B cells results in surface expression of μH chain in the form of a complex with VpreB and λ5, called the pre–B-cell receptor (pre-BCR), resulting in differentiation to the pre–B-cell stage. Transient surface expression of the pre-BCR triggers rapid cell-cycle progression, thereby forming a large pre–B-cell population, and ultimately promoting development toward the small pre–B-cell and immature B-cell stages.1,2 Pre–B cells in the absence of signals derived from the pre-BCR undergo apoptotic cell death.3 Signal transduction from the pre-BCR requires recruitment and activation of the Syk tyrosine kinase.4 Activated Syk phosphorylates several downstream signaling elements, including BLNK (also known as SLP-65 or BASH)
BLNK is a pivotal adapter protein in signal transduction from the pre-BCR and BCR. BLNK contains multiple tyrosine phosphorylation sites that provide binding sites for key signaling proteins such as PLCγ, Btk, and Vav.5 BLNK gene mutations cause a complete block in B-cell development at the pro–B-cell to pre–B-cell transition in humans.6 In BLNK-null mutant mice the developmental block is partial, resulting in the accumulation of pre-BCR+ large pre–B cells in the bone marrow and a reduction of mature B cells in the periphery.7 We and others previously reported that 5% to 10% of BLNK−/− mice spontaneously develop pre–B-cell leukemia at 4 to 20 weeks of age.7–9 Pre–B-cell–derived acute lymphoblastic leukemia (pre–B-ALL) is the most common type of leukemia in children.10 Interestingly, one study reported that 50% of the pediatric B-ALL cases they investigated had lost BLNK protein expression,11 although other studies reported a lower frequency.12,13 Thus, it has been proposed that BLNK functions as a tumor suppressor, but the molecular mechanisms by which it exerts tumor suppressor activity are still unknown. Because tumorigenesis is a multistep process requiring sequential changes in various genes,14 it is unlikely that BLNK deficiency is sufficient to initiate leukemogenesis.
Combined deficiency of BLNK and Btk results in a more severe developmental block at the pre–B-cell stage15 and a higher incidence of pre–B-cell leukemia compared with mice deficient in either gene alone,7–9,16 suggesting that the developmental block is one of the tumor-promoting factors. However, mice that cannot express the pre-BCR, such as μMT or RAG-deficient mice, exhibit a complete developmental block at the pro-B stage but do not develop leukemia.17 These results indicate that surface expression of the pre-BCR is essential for the development of leukemia. In Btk/PLCγ2 and IRF4/IRF8 double-deficient mice, a nearly complete block of early B-cell development resulted in an accumulation of pre-BCR+ cycling pre–B cells, but so far development of pre–B-cell leukemia has not been reported.18,19 Thus, in addition to the developmental arrest at the pre–B-cell stage and pre-BCR expression, a defect in BLNK-specific function seems to be required for pre–B-cell leukemogenesis.
The expansion of pre–B cells in the bone marrow depends not only on pre-BCR signaling but also on IL-7 secreted from stromal cells.20,21 Involvement of IL-7 in pre–B-cell leukemogenesis has been suggested by the experiments showing that mice overexpressing transgenic IL-7 or administered with exogenous IL-7 exhibited a significant increase in B-cell progenitors and eventually onset of B leukemia/lymphoma.22–24 In addition, constitutive activation of STAT5 induced by retrovirus integration was found in some pre–B-cell lymphomas that developed in mice of a lymphoma-prone strain.25 Previously, it was reported that pre-BCR expression increases the sensitivity of pre–B cells to IL-7, which enhances the proliferation rate of pre–B cells in vitro as well as in vivo26–28; this is due, at least partly, to pre-BCR–dependent, but BLNK-independent, enhancement of cyclin D3 stability.29 The pre-BCR+ pre–B cells that accumulate in BLNK−/− mice in vivo show a smaller percentage of cells in S/G2/M phases of the cell cycle when compared with wild-type large pre–B cells.9 However, ex vivo BLNK−/− pre–B cells display a markedly enhanced proliferative response to IL-7 when compared with wild-type pre–B cells in vitro.8 Thus, the role of BLNK in pre-BCR signaling that regulates pre–B-cell proliferation is still enigmatic, and the mechanism for BLNK tumor suppression remains unclear. Here, we show that constitutive activation of the JAK3/STAT5 signaling pathway leads to suppression of p27kip1 expression and plays an essential role in the autonomous proliferation of pre-B leukemia cells that developed in BLNK−/− mice. Activation of the JAK3/STAT5 pathway is partly due to autocrine production of IL-7. Moreover, in vivo constitutively active STAT5 synergized with BLNK deficiency to induce leukemogenesis in the mice. Finally, we show that BLNK inhibits JAK3 activation through direct interaction with JAK3 and thus suppresses cell-cycle progression and growth.
Methods
Mice
BLNK(BASH)−/− mice9 and STAT5b-CA transgenic mice30,31 were described previously. All experiments involving mice were approved by the Institutional Review Board of the Tokyo University of Science.
Cell culture
Pre-B leukemia cell lines derived from BLNK−/− (BKO series), BLNK−/− CD19−/− (DKO series), and BLNK+/−STAT5b-CA mice were generated by long-term culture of bone marrow cells in RPMI-1640 medium containing fetal bovine serum (FBS) and antibiotics without any other additives, as described.9 Primary bone marrow pre–B cells from unaffected mice were cultured for 4 days in Iscove medium containing 20% FBS, 5 ng/mL recombinant IL-7 (PeproTech, Rocky Hill, NJ), and 100 U/mL penicillin and streptomycin. To measure cell growth rates, the numbers of living cells were counted after staining with Trypan Blue (GIBCO [now Invitrogen, Carlsbad, CA]).
Antibodies
Anti–IL-7R monoclonal antibody was purified from ascites fluid of mice injected with A7R34 hybridoma.32 Rabbit anti-BLNK antibody was described previously.33 Antibodies against phospho-JAK3, STAT5a/b, p27kip1, Erk1/2, Btk, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); anti–phospho-STAT5a/b, anti–phospho-AKT, and anti–phospho-Erk1/2 antibodies were purchased from Cell Signaling (Danvers, MA); anti-JAK3 antibody from Upstate Biotechnology (Charlottesville, VA); and anti-SOCS1 from Zymed (South San Francisco, CA).
Western blot analysis and Immunoprecipitation
Protein concentration in cell lysate was quantified by the Bradford method using Protein Assay (Bio-Rad, Hercules, CA). Each lysate equivalent to 100 μg of protein was resolved by 6% or 10% SDS-PAGE and transferred to PVDF membranes (GE Healthcare, Little Chalfont, United Kingdom). Western blotting was performed as described previously.33 Immunoprecipitation was performed as described,34 except that TrueBlot (eBioscience, San Diego, CA) was used for the secondary antibody in immunoblotting of the precipitates.
Flow cytometry
Cells were stained with FITC-conjugated anti-IgD (BD Pharmingen, San Diego, CA), PE-conjugated anti-IgM (Southern Biotechnology, Birmingham, AL), PE-conjugated anti-B220 (eBioscience), biotin-conjugated anti-SL156 (BD Pharmingen), biotin-conjugated anti–IL-7Rα chain (BD Pharmingen) or annexin-V–biotin (Boehringer Mannheim [now Roche Diagnostics, Basel, Switzerland]). Flow cytometry was performed as described33 and analyzed with FlowJo software (TreeStar, Ashland, OR). Cell-cycle profile was analyzed as described.33
Plasmids and retroviral infection
The retroviral vectors pMX-IRES-GFP,35 pMX-IRES-rCD2,34 pMX-SOCS1-IRES-GFP,36 and pMX-SOCS1 F59D-IRES-GFP36 were described previously. STAT5a and dnSTAT5a cDNAs37 were cloned into pMX-IRES-rCD2 vector. The mutant mouse BLNK cDNAs containing tyrosine to phenylalanine substitutions were generated by polymerase chain reaction (PCR)–mediated mutagenesis and cloned into pMX-IRES-rCD2 vector. These vectors were transfected into the PLAT-E packaging cell line, and the supernatant was used for infection as described.34 The cells infected with pMX-IRES-rCD2–based vectors were sorted by a magnetic-activated cell sorter (MACS) system as described previously.34
RT-PCR
cDNA was generated from total RNA as described.34 The cDNA was amplified by PCR with the following primers: IL-7, 5′-ATCTTTGGAATTCCTCCACT-3′ and 5′-GCCCTTCAAAATTTTATTCC-3′; HPRT, 5′-TTGCTGGTGAAAAGGACCTCTCG-3′ and 5′-CCACAGGACTAGAACACCTGCTAA-3′; BLNK, 5′-ATGGACAAGCTGAA-3′ and 5′-TTATGAAACCTTCA-3′; p27kip1, 5′-ATGTCAAACGTGAGAGTGTC-3′ and 5′-TTACGTCTGGCGTCGAAGGC-3′; and GAPDH, 5′-CCAAGGTCATCCATGACAAC-3′ and 5′-CTGTTGCTGTAGCCGTATTC-3′. For semiquantitative reverse transcription (RT)–PCR, cDNA samples were serially diluted 4-fold before amplification. cDNA quantity among the samples was normalized based on the data of PCR using the serially diluted GAPDH cDNA.
Retrovirus-mediated siRNA
The following sequences were selected as the targets of short-hairpin (sh) RNA: Btk, 5′-GGGAAAGAAGGAGGTTTCATT-3′; p27kip1, 5′-CGCAAGTGGAATTTCGACTTT-3′; and luciferase, 5′-GTGCGTTGCTAGTACCAA-3′. The shRNA-expressing retrovirus vectors were constructed using RNAi-Ready pSIREN-RetroQ vector (Clontech, Mountain View, CA) according to the supplier's instruction.
Results
BLNK−/− pre-B leukemia cells acquire autonomous activation of JAK3/STAT5 signaling triggered by autocrine IL-7 production
We first tested the possibility that activation of IL-7R-JAK-STAT5 signaling may contribute to the generation of pre–B-cell leukemia in BLNK-deficient mice. Accordingly, we examined the cells from enlarged lymph nodes (LNs) of BLNK−/− mice suffering from leukemia. We found that the activation-associated phosphorylation of JAK3 and STAT5 was enhanced when compared with pre–B cells from unaffected BLNK−/− mice (Figure 1A). Although it was previously reported that Erk activity is necessary for survival of an IL-7–dependent pre–B-cell line from another strain of BLNK-deficient mice,8 Erk activity was much lower in the leukemic LN samples than in the nontransformed BLNK−/− pre–B cells (Figure 1A).
Figure 1.
Constitutive activation of JAK3/STAT5 signaling by autocrine IL-7 expression confers proliferation ability on BLNK−/− pre-B leukemia cells. (A) Activation state of JAK3, STAT5a/b, and Erk1/2 in primary BLNK−/− pre-B leukemia cells (top panel) and of STAT5a/b in BLNK-pBLs (bottom panel) was assessed by Western blotting of the cell lysates with antibodies against an activation-coupled phosphorylated site in each protein. The same filters were reblotted with the antibodies against each protein. BM indicates pre–B cells purified from bone marrow cells of BLNK−/− mice that did not develop the leukemia. LN1–3 indicates pre-B leukemia cells purified from enlarged lymph nodes of 3 individual BLNK−/− mice. (B) BKO84 cells were transduced with either SOCS1 or SOCS1 F59D via retrovirus vectors containing EGFP as a selection marker. EGFP fluorescence in the infected (open) and nontreated (shaded) cells at the indicated times after infection was analyzed by flow cytometry. The percentage of EGFP+ cells in each infected sample is indicated. (C) RT-PCR analysis of IL-7 mRNA in primary BLNK−/− pre-B leukemia cells (left panel; the same samples as in panel A), BLNK-pBLs, A-MuLV–transformed pre–B-cell line 18-81, B-cell lymphoma line WEHI231, and IL-7–producing stromal cell lines ST2 and OP9 (right panel). HPRT mRNA was amplified to control cDNA quantity. (D) Growth curves of BLNK-pBLs and 18-81 cultured in the medium containing αIL-7R (■), class-matched control antibody (▵), or none (○) are shown. Each symbol represents mean numbers (± SD) of triplicate cultures. All data except panel A (top) are representative of 3 independent experiments.
To investigate if activated JAK3/STAT5 signaling caused the autonomous growth of the leukemic cells, we examined BLNK-deficient pre-B leukemia cell lines (BKO and DKO series; together called BLNK-pBLs hereafter) derived from bone marrow cells of BLNK−/− and BLNK−/− CD19−/− mice, respectively.34 Each line was derived from an affected mouse and was monoclonal as assessed by unique IgH gene rearrangements,9 and expressed pre-BCR homogeneously (data not shown). Karyotype analyses confirmed by fluorescence in situ hybridization (FISH) with chromosome specific probes revealed no evidence of interchromosomal translocation; however, trisomies and a segmental inverted duplication of each different chromosomes were found in 3 of the BLNK-pBLs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, no gross recombination was detected by Southern blot analysis in either E2A or c-myc gene loci (Figure S2). Western blot analysis revealed that STAT5 is activated in these cell lines (Figure 1A bottom panel; others not shown). Since they were established and maintained without any cytokines, these data suggest autonomous activation of STAT5 or upstream factors involved in STAT5 signal transduction. JAK activity appeared to be necessary for their growth since retroviral expression of functional SOCS1, a physiologic inhibitor of JAK kinases but not a nonfunctional mutant (SOCS1 F59D),36 resulted in elimination of the infected cells marked with enhanced green fluorescent protein (EGFP) in BKO84 (Figure 1B) and other cell lines (data not shown). In addition, transduction of a dominant-negative STAT5 mutant into BKO84 cells also eliminated the infected cells (data not shown). These results indicate that constitutive activation of JAK3/STAT5 signaling endows the BLNK-pBLs with autonomous growth competence.
We next investigated the mechanism for the constitutive activation of JAK3/STAT5 signaling in these cells. We found that 2 of the 3 LN pre–B-cell samples, each derived from individual leukemic mice, and all of the BLNK-pBLs constitutively expressed IL-7 mRNA (Figure 1C). To test whether the BLNK-pBLs indeed secrete IL-7 and require IL-7–induced signals to proliferate, they were cultured in medium containing anti–IL-7R–blocking antibody (αIL-7R).32 The growth of all the cell lines except DKO35 was blocked by the αIL-7R, but not by an isotype-matched control antibody (Figure 1D), indicating that their growth is dependent on autocrine production of IL-7. As a control, the growth of 18-81 cells was not affected by the αIL-7R. DKO35 cells might have acquired a mutation that allows IL-7–independent JAK activation. Taken together, these results indicate that somatic, genetic (or epigenetic) lesions that cause constitutive activation of the JAK3/STAT5 signaling pathway (frequently through constitutive expression of IL-7 in pre–B cells) significantly contribute to leukemogenesis of BLNK-deficient pre–B cells in mice.
Inhibition of JAK3/STAT5 signaling induces p27kip1 expression and G1 cell-cycle arrest in BLNK-pBLs
We next investigated the mechanism that underlies the unregulated growth of the BLNK-pBLs. Cultivation of BKO84 cells with αIL-7R for 48 hours resulted in cell-cycle arrest in G1 and a modest appearance of subdiploid apoptotic cells (Figure 2A). As it was previously reported that IL-7 regulates expression of the cyclin-dependent kinase inhibitor (CDKI) p27kip1 in T cells,38 we examined the expression levels of p27kip1 in BKO84 cells in a time course following addition of αIL-7R. Western blotting and semiquantitative RT-PCR analysis revealed that IL-7R blockade markedly up-regulated p27kip1 expression at both protein and mRNA levels (Figure 2B). Among other CDKIs, p21cip1 mRNA level was unchanged, while expression of p16INK4a and p19Arf was undetectable during the time course. In addition, mRNA levels of cyclin D2 and cyclin D3 were also unchanged (data not shown). The same G1 cell-cycle arrest with p27kip1 up-regulation was also observed in other αIL-7R–sensitive BLNK-pBLs, BKO408 and DKO18 (data not shown). These results indicate that IL-7R signaling selectively down-regulates p27kip1 expression at the RNA level.
Figure 2.
Constitutive activation of JAK3/STAT5 signaling inhibits p27kip1 expression in BKO84 cells. (A) Cell-cycle profiles of BKO84 cells that were cultured in medium containing αIL-7R for the indicated time period. Cells were stained with propidium iodide and analyzed by flow cytometry. Percentages of cells in subdiploid (if present), G1, and S/G2/M phases are indicated by numbers. (B) Western blot analysis of p27kip1 protein (top) and β-actin as a loading control (top middle), and semiquantitative RT-PCR analysis of p27kip1 mRNA (bottom middle) and GAPDH as a cDNA quantity control (bottom) of the same cells as in panel A. (C,D) Cell-cycle analysis (C) and Western blot analysis for p27kip1 protein (D) in BKO84 cells infected with retroviral vectors carrying either dnSTAT5a or STAT5a coupled via an IRES to rCD2. Cells expressing the rCD2 were magnetically sorted at the indicated times after infection and analyzed. (E) Semiquantitative RT-PCR analysis of p27kip1 mRNA and GAPDH mRNA (cDNA quantity control) in BKO84 cells transduced with dnSTAT5a as in panel D. All data are representative of 3 independent experiments.
We next tested whether the JAK3/STAT5 signaling pathway is involved in the regulation of p27kip1. BKO84 cells were infected with retroviral vectors carrying an IRES-linked rat CD2 (rCD2) as an infection marker, and wild-type STAT5a or a dominant-negative form of STAT5a (dnSTAT5a) that lacks a C-terminal transcriptional activation domain and blocks STAT5 activity altogether.37 After 24 and 48 hours of infection, rCD2+ cells were magnetically sorted, and their DNA content and p27kip1 expression were analyzed by flow cytometry and Western blotting, respectively. dnSTAT5a expression in BKO84 cells induced G1 cell-cycle arrest and up-regulation of p27kip1 protein level 48 hours after infection (Figure 2C,D). dnSTAT5a expression also increased p27kip1 mRNA levels (Figure 2E). These results indicate that autonomous activation of JAK3/STAT5 signaling pathway promotes cell-cycle progression into S phase by inhibiting p27kip1 expression.
Loss of BLNK coupled with STAT5 activation synergistically accelerates pre–B-cell leukemogenesis
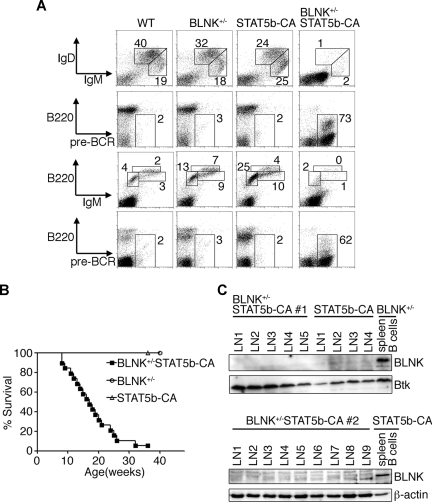
The above results suggest that autonomous activation of the JAK3/STAT5 signaling pathway may have conferred growth competence on the BLNK−/− pre–B cells to generate these leukemia cells. To prove this, we introduced a transgene expressing STAT5b-CA into BLNK-deficient mice by intercrossing the STAT5b-CA transgenic30 and BLNK−/− mice. Previous reports have documented that expression of the STAT5b-CA transgene can be detected in pro–B cells (both mRNA and protein) and is maintained throughout B-cell development.30,31 To our surprise, 32 of 36 heterozygous BLNK-mutant mice carrying the STAT5b-CA transgene exhibited multiple enlarged LNs 16 weeks after birth. Flow cytometric analysis of the affected mice revealed that pre-BCR+ leukemic cells dominated in spleen, bone marrow, LN, and peripheral blood (Figure 3A; data not shown). PCR analysis of rearranged Ig heavy-chain variable region genes revealed that the leukemic cells in multiple LNs in a mouse originated from at least 3 distinct pre–B-cell clones (data not shown). Oligoclonality of the leukemia is also supported by a frequent appearance of mixed leukemia cell populations with different levels of B220 expression in a mouse (Figure 3A; data not shown). As demonstrated in the Kaplan-Meier survival curves (Figure 3B), 97% of BLNK+/−STAT5b-CA mice developed leukemia and died within 36 weeks after their birth. In parallel experiments, only one of 60 STAT5b-CA mice developed leukemia within 36 weeks, which is a similar frequency to that reported previously (1%-2%).30 No BLNK+/− mice (n = 16) developed leukemia within 12 months after their birth.
Figure 3.
Transgenic expression of constitutively active STAT5 accelerates pre–B-cell leukemogenesis in BLNK+/− mice. (A) Flow cytometric analysis of nucleated cells of spleens (panels in top 2 rows) and bone marrows (panels in bottom 2 rows) from mice of the indicated genotypes. Numbers indicate percentage of cells falling in each window. Data are representative of 3 independent experiments. (B) Kaplan-Meier curves depicting the proportion of mice that remain alive with age. BLNK+/− (○), STAT5b-CA (▵), and BLNK+/−STAT5b-CA (■) mice are shown. (C) Western blot analysis for BLNK and Btk (top) or β-actin (bottom) proteins in primary pre-B leukemia cells from individual lymph nodes (LN#) in 2 BLNK+/−STAT5b-CA mice (#1 and #2; top and bottom, respectively) or a STAT5b-CA mouse (top). As controls, proteins from MACS-purified spleen B cells from unaffected BLNK+/− (top) or STAT5b-CA (bottom) mouse are included.
We next investigated why BLNK+/− mice, which are nonleukemogenic by themselves, developed leukemia upon expression of the STAT5b-CA transgene. Western blot analysis of markedly enlarged LNs from affected BLNK+/− STAT5b-CA mice and from one affected STAT5b-CA mouse revealed that BLNK protein expression was almost extinguished in the cells of these LNs compared with that in purified splenic B cells from BLNK+/− or unaffected STAT5b-CA mice (Figure 3C). RT-PCR analysis revealed that BLNK mRNA expression level was extremely low in the same LNs from BLNK+/− STAT5b-CA mice (Figure S3). Preliminary sequencing data indicated the presence of the abnormally spliced BLNK mRNAs resulting in the exon-skipping and frame shifts, which may account for the low expression (data not shown). These data indicate that somatic gene modification(s), resulting in the silencing of BLNK mRNA and protein expression, cooperate with STAT5b-CA to induce transformation of pre–B cells.
BLNK negatively regulates the JAK3/STAT5 signaling pathway
These results suggest that BLNK suppresses the transforming activity of STAT5b-CA in pre–B cells, since the incidence of leukemia was very low in BLNK-sufficient STAT5b-CA mice. In accord with this, BKO84 and other BLNK-pBL cells reconstituted with BLNK could not grow in vitro (data not shown), and underwent G1 cell-cycle arrest with modest apoptosis as represented by subdiploid cell fractions (Figure 4A). Western blotting of the sorted BLNK-reconstituted BKO84 cells revealed that BLNK expression resulted in deactivation of JAK3 and STAT5 as assessed by dephosphorylation of active sites within 48 hours. In addition, marked elevation of p27kip1 protein levels were observed 96 hours after infection (Figure 4B). This was not attributable to down-regulation of surface IL-7R expression (Figure 4C) or up-regulation of SOCS1 (Figure 4B). BLNK expression caused down-regulation of surface pre-BCR expression as reported previously (Figure 4C).8,34 However, activation levels of Erk1/2 and AKT were comparable between the cells infected with mock- and BLNK-retroviral vectors (Figure 4B). These data indicate that BLNK negatively regulates the JAK3/STAT5 signaling pathway, which leads to up-regulation of p27kip1, culminating in G1 cell-cycle arrest. The same appears to hold true of primary pre–B cells cultured in vitro with IL-7: compared with wild-type (WT) pre–B cells, BLNK−/− pre–B cells grow faster8 (data not shown), exhibit stronger phosphorylation of JAK3 and STAT5, and diminished amounts of p27kip1 expression (Figure 4D).
Figure 4.
BLNK negatively regulates the JAK3/STAT5 signaling pathway in pre-B leukemia cell lines and primary pre–B cells. (A-C) BKO84 cells were infected with retroviral vectors carrying either BLNK cDNA or no cDNA insert (MOCK) coupled via an IRES to rCD2. rCD2+ cells were sorted by MACS 48, 72, and 96 hours after infection and used for each analysis. (A) Cell-cycle analysis was done as in Figure 2A. (B) Cell lysates were analyzed by Western blot analysis with the indicated antibodies. (C) Surface expression of IL-7Rα and pre-BCR in rCD2− cells (solid line) and rCD2+ cells (dotted line) were analyzed by flow cytometry. Nonstained controls are shown in gray. (D) κ− λ− B220+ pro/pre–B cells were purified from the bone marrow of WT or BLNK−/− mice by MACS sorting, cultured in the medium containing IL-7 for 96 hours, lysed, and then analyzed by Western blot analysis with the indicated antibodies. All data are representative of 3 independent experiments. The reason for the increased phospho-ERK1/2 in both mock- and BLNK-introduced cells (panel B; see also Figure 6B) is unclear, but it might be due to the stresses that the cells might have suffered during the retroviral infection. (E,F) Parental, si-Luc, or si-p27kip1 BKO84 cells were transduced with BLNK and rCD2+ cells were sorted at the indicated time points as in panels A to C, and analyzed by Western blotting (E) or for cell-cycle profiles (F), as in panels B or A, respectively.
To determine whether the up-regulation of p27kip1 is responsible for the BLNK-mediated cell-cycle inhibition in the pre-B leukemia cells, we generated stable subclones of BKO84 in which p27kip1 expression was suppressed (si-p27kip1 BKO84) and an irrelevant luciferase was targeted (si-Luc BKO84) through small interfering RNAs (siRNAs). In the si-p27kip1 BKO84 cells, retroviral vector–mediated BLNK reconstitution resulted in the deactivation of JAK3 and STAT5 as in the control si-Luc BKO84cells, but p27kip1 expression was poorly induced as expected (Figure 4E). In the BLNK-reconstituted si-p27kip1 BKO84 cells, cell cycle was only marginally inhibited compared with the control si-Luc BKO84 cells even 96 hours after infection, but apoptosis was induced instead (Figure 4F). This result indicates that p27kip1 up-regulation is necessary for BLNK to inhibit cell-cycle progression in BKO84 cells; therefore, p27kip1 is a key regulator of cell cycle in the pathways downstream of JAK3 and STAT5 in BKO84 cells.
BLNK inhibits JAK3 activity and induces apoptosis in the presence of STAT5b-CA
These results suggest that BLNK inhibits JAK3, an upstream kinase for STAT5, or further upstream molecules in the IL-7R signaling pathway. To clarify how the loss of BLNK synergizes with STAT5b-CA to transform pre–B cells in BLNK+/−STAT5b-CA mice, we established a pre–B-cell line from bone marrow cells of such mice suffering from leukemia. In this cell line, IL-7 mRNA was constitutively expressed and JAK3 was constitutively activated, similar to the BLNK-pBLs (Figure 5A,E). In addition, BLNK expression was extinguished (Figure 5E), as in the primary leukemia cells that developed in other mice (Figure 3C). These data suggest that constitutive activation of JAK3 provided unknown downstream signals that are required in addition to STAT5b-CA expression for transformation of pre–B cells in mice. To clarify the role of JAK3 in the autonomous growth of the BLNK+/−STAT5b-CA cell line, these cells were transduced with retrovirus vectors expressing either SOCS1 or BLNK, or SOCS1 F59D (Figure 5B). As demonstrated by the reduced proportion of EGFP+ cells, the cells expressing SOCS1 or BLNK did not grow in culture; in contrast, cells expressing the SOCS1 F59D continued to proliferate. BLNK reconstitution in the BLNK+/−STAT5b-CA cells did not induce a significant G1 cell-cycle arrest in these cells 72 and 96 hours after infection, but induced marked apoptosis, similar to that induced by SOCS1 (Figure 5C). BLNK- and SOCS1-induced apoptosis was also demonstrated by the marked increase of annexin-V–stained cells (Figure 5D). Finally, BLNK expression reduced the activity of JAK3 by 72 hours after infection, whereas phosphorylation of STAT5 was unchanged (Figure 5E). These data can be interpreted as indicating that BLNK inhibited JAK3 activity and its downstream antiapoptotic signaling pathway,39 whereas a distinct downstream JAK3 signaling pathway involving STAT5 activation was provided by the STAT5b-CA transgene; therefore, p27kip1 expression and cell-cycle arrest were not induced. These results suggest that the loss of BLNK augments JAK3 activation induced by IL-7 and the following activation of downstream pathways, leading to antiapoptosis and STAT5-mediated cell-cycle progression. Transgenic STAT5b-CA synergizes with this effect of BLNK on leukemogenesis by boosting cell-cycle progression and thus increasing the probability that cells acquire additional genetic modifications that promote constitutive JAK3 activation.
Figure 5.
BLNK expression in BLNK+/−STAT5b-CA pre-B leukemia cell line induces apoptosis. (A) RT-PCR analysis of IL-7 and GAPDH (cDNA quantity control) mRNA expression in the indicated cell lines. (B) The BLNK+/−STAT5b-CA pre-B leukemia cell line was infected with retroviral vectors carrying either SOCS1, SOCS1 F59D, or BLNK, coupled via an IRES to EGFP. EGFP expression in the virus-infected cells (open) and noninfected cells (shaded) was analyzed by flow cytometry. The percentage of EGFP+ cells in each infected sample is indicated. (C-E) BLNK+/−STAT5B-CA pre-B leukemia cell line was infected with retroviral vectors carrying either SOCS1, SOCS1 F59D, BLNK, or none (MOCK), coupled via an IRES to rCD2. rCD2+ cells were sorted by MACS 72 and 96 hours after infection and used for each analysis. (C) Cell-cycle profiles of SOCS1, BLNK, or MOCK-transduced cells were analyzed as in Figure 2A. (D) Sorted cells were stained with annexin-V and analyzed by flow cytometry. The mean (± SD) proportion of apoptotic cells (Annexin-V+) in duplicate samples is indicated by each bar. (E) Lysates of BLNK- or MOCK-transduced cells were analyzed by Western blotting with the indicated antibodies. All data are representative of 2 independent experiments.
BLNK mediated down-regulation of JAK3/STAT5 signaling is independent of Btk
It was reported that BLNK deficiency synergizes with Btk deficiency in mouse leukemogenesis.15 In addition, reconstitution of BLNK, but not of the BLNK mutant with a tyrosine-replacement (Y96F) at a proposed Btk-binding site, in a BLNK−/− pre–B-cell line prevented its growth in vivo.11 Finally, bone marrow cells from Btk−/− mice show enhanced proliferative responses to IL-7 to the same extent as those from BLNK−/− mice.15 These reports suggest that BLNK might cooperate with Btk in the suppression of the JAK3-STAT5 signaling pathway required for pre–B-cell growth. To test this, we generated a Btk-deficient BKO84 subclone (si-Btk BKO84) through infection of a siRNA-expressing retroviral vector. BLNK reconstitution induced G1 cell-cycle inhibition in the control si-Luc BKO84 cells, but had only a marginal effect in the si-Btk BKO84 cells 96 hours after infection. Instead, BLNK expression induced marked apoptosis in the si-Btk BKO84 cells (Figure 6A). In accord with this, BLNK reconstitution did not induce p27kip1 protein expression in the si-Btk BKO84 cells, unlike the parental and the si-Luc BKO84 cells (Figure 6B). However, JAK3 and STAT5 were clearly deactivated (Figure 6B), and the expression of p27kip1 mRNA was up-regulated (Figure 6C) by BLNK reconstitution in the si-Btk BKO84 cells as much as in the Btk-sufficient BKO84 or si-Luc BKO84 cells. These results indicate that BLNK suppresses JAK3 activity, leading to STAT5 deactivation and up-regulation of p27kip1 mRNA levels, in a Btk-independent manner. However, the accumulation of p27kip1 protein and cell-cycle arrest is dependent on Btk. In this respect, Btk might facilitate synthesis or stabilization of the p27kip1 protein. Thus, BLNK and Btk may suppress the IL-7R–signaled pre–B-cell growth by acting at distinct levels; this finding may explain how the loss of BLNK and Btk synergistically increases the incidence of pre–B-cell leukemia. This result also supports the notion that p27kip1 is the key regulator of cell cycle in the pathways downstream of JAK3 in BKO84 cells.
Figure 6.
BLNK-inhibition of JAK3/STAT5 signaling pathway is Btk independent, but induction of p27kip1 protein and cell-cycle arrest are Btk dependent in BKO84 cells. (A-C) BKO84, si-Luc BKO84, and si-Btk BKO84 cells were infected with a retroviral vector carrying BLNK coupled via an IRES to rCD2, and rCD2+ cells were sorted by MACS 72 and 96 hours after the infection and used for each analysis. (A) Cell-cycle profiles of the BLNK-transduced si-Luc BKO84 and si-Btk BKO84 cells were analyzed as in Figure 2A. (B) Cell lysates were analyzed by Western blotting with the indicated antibodies. (C) Semiquantitative RT-PCR analysis of p27kip1 mRNA and GAPDH mRNA (cDNA quantity control) in BLNK-transduced si-Btk BKO84 and si-Luc BKO84 cells 72 hours after infection. All data are representative of 2 independent experiments.
Binding of BLNK to JAK3 is required for BLNK-mediated inhibition of JAK3 activity and growth of pre–B cells
So far, BLNK is the only known tumor suppressor among the pre-BCR signaling factors whose loss alone renders mice leukemogenic. Therefore, we reasoned that BLNK is primarily responsible for JAK3 inhibition. To address whether BLNK directly interacts with JAK3, BLNK was reconstituted in BKO84 cells via a retrovirus vector, and the cell lysate was subjected to an immunoprecipitation assay. As shown in Figure 7A, JAK3 coprecipitated with BLNK when using an anti-BLNK antibody, and BLNK was coprecipitated by JAK3 when using an anti-JAK3 antibody, indicating that BLNK and JAK3 interact in this cell line. Since a BLNK mutant Y96F failed to suppress pre–B-cell growth in vivo,11 we examined whether this tyrosine is necessary for the binding of BLNK and JAK3. As shown in Figure 7C, the BLNK Y96F mutant (Figure 7B) expressed in BKO84 cells exhibited significantly weaker binding with JAK3 compared with unmutated BLNK. Another mutant of BLNK, YF3 (Figure 7B), containing further substitutions of phenylalanine for the tyrosines adjacent to the tyrosine 96 (Y84 and Y119), hardly bound to JAK3. In contrast, the mutants containing either single tyrosine substitution (Y84F or Y119F) had no effect on the JAK3 binding (Figure 7C). This result indicates that the tyrosine 96 is critical for efficient BLNK binding to JAK3, whereas the adjacent tyrosines (84 and 119) partially compensate for the lack of the tyrosine 96 in this binding. Finally, we examined whether these mutations affect BLNK inhibition of JAK3/STAT5 signaling and pre–B-cell growth. Upon expression in BKO84 cells via retrovirus vectors, BLNK and Y84F strongly inhibited activation of JAK3 and STAT5 and induced p27kip1 protein expression. However the inhibition of JAK3/STAT5 activity was delayed in the case of Y96F, and was abolished in YF3. Accordingly, p27kip1 protein expression was only weakly induced by Y96F, and not at all by YF3 (Figure 7D). Such changes of JAK3/STAT5 activities and p27kip1 protein levels showed a perfect correlation with the extent of cell-cycle arrest: upon expression in BKO84 cells, G1 cell-cycle arrest was not induced by YF3 and less so by Y96F compared with WT BLNK and Y84F (Figure 7E). These results indicate that BLNK inhibits JAK3/STAT5 signaling and cell-cycle progression in a manner that depends on binding to JAK3.
Figure 7.
BLNK binding with JAK3 is essential for the inhibition of JAK3/STAT5 signaling, leading to cell-cycle arrest and apoptosis. (A) BKO84 cells were infected with a retroviral vector carrying BLNK. At 48 hours later, these cells were lysed and immunoprecipitated with αBLNK antibody or a control rabbit IgG (center panels), or with αJAK3 antibody or a control mouse IgG (right panels). These precipitates and the lysates (left panels) were analyzed by Western blotting with αJAK3 (top) or αBLNK (bottom) antibodies. (B) Tyrosine-to-phenylalanine mutants of BLNK. The numbers indicate the positions of tyrosine (Y) or substituted phenylalanine (F) residues in BLNK or BLNK mutants. Other single Y-to-F mutants, Y84F and Y119F, were not depicted. (C-E) BKO84 cells were infected with retroviral vectors carrying either BLNK or one of the BLNK mutants coupled via an IRES to rCD2. (C) Cells were lysed 48 hours after infection, immunoprecipitated with αBLNK antibody, and then blotted with αJAK3 (top) or αBLNK (middle) antibodies. The cell lysates were blotted with αJAK3 antibody (bottom). (D,E) At 72 and 96 hours after infection, rCD2+ cells were sorted by MACS. The sorted cells were lysed, and the lysates were analyzed by Western blotting with the indicated antibodies (D). Cell-cycle profiles of the sorted cells were analyzed as in Figure 2A (E). All data are representative of 2 independent experiments.
Discussion
BLNK has recently been recognized as a tumor suppressor in pre–B-cell leukemogenesis, but the mechanism for how BLNK exerts its tumor-suppressor function remains unknown. It has been particularly mysterious how the signaling protein required for pre-BCR– and BCR-mediated cell-cycle progression in vivo suppresses both in vitro pre–B-cell proliferation and leukemogenesis. In this study, we demonstrate that autonomous activation of JAK3/STAT5 coordinates with BLNK deficiency in the unregulated growth of BLNK-pBLs, and pre–B-cell leukemogenesis in BLNK+/−STAT5b-CA mice. Moreover, we demonstrate that BLNK binds to and inhibits JAK3, and thereby inhibits cell-cycle progression of the BLNK-pBLs. Thus, BLNK appears to suppress leukemogenesis in mice at 2 phases: first, BLNK initially regulates the physiologic IL-7–mediated expansion or survival of large pre–B cells and thereby reduces the probability of somatic gene lesions; second, BLNK suppresses clonal proliferation of leukemic cells induced by mutations such as ectopic IL-7 expression that drive constitutive activation of JAK3. Finally, our data indicate that reversion of the JAK3/STAT5-mediated repression of p27kip1 expression is critical for BLNK-mediated suppression of proliferation in the second phase.
In this study, we have identified JAK3 as a novel binding partner of BLNK and shown that this binding is essential for inhibition of pre-B leukemia cell growth mediated by JAK3/STAT5 signaling. Because Y96 of BLNK is crucial for this interaction, with some compensation by Y84 and Y119, it is likely that phosphorylation of BLNK on these tyrosines is required. Syk is generally believed to be responsible for BLNK phosphorylation, and therefore is likely to positively regulate the BLNK binding with JAK3. However, pre-BCR signaling through Syk is not inhibitory, but actually promotes the expansion of large pre–B cells by cooperating with IL-7 signaling.26,40 Given that Syk promotes BLNK-dependent inhibition of JAK3, how does the pre-BCR signal to promote pre–B-cell expansion? Several models may explain this. The first model assumes that pre-BCR signaling induces de novo expression of a negative regulator of JAK3 that is recruited to JAK3 via BLNK. In this model, IL-7R signaling would continue to promote proliferation of pre–B cells until the regulator protein accumulates to levels sufficient to inhibit JAK3 activity. Here, BLNK would function as an adaptor to recruit a negative regulator yet to be identified. We have tested a possible involvement of the previously reported JAK3 phosphatases CD45, SHP-1, or TCPTP41 through an siRNA-mediated knockdown approach, but reduction of any of these proteins did not affect BLNK-mediated deactivation of JAK3 in BKO84 cells. Thus, the negative regulator model remains to be proven.
The second model proposes a mechanism where surface pre-BCR expression controls the intracellular localization of BLNK. BLNK is held at the membrane close to the pre-BCR when the pre-BCR is expressed on the cell surface, perhaps through binding to Ig-α,42 and phosphorylated by Syk. When surface pre-BCR expression is down-regulated, the phosphorylated BLNK is released, moves to JAK3 in the IL-7R signalosome, and inhibits JAK3. Indeed, the surface pre-BCR level correlated with JAK3 activity in the time course after induction of BLNK into BKO84 cells (Figure 4B,C; data not shown). In an experiment using BKO84 cells carrying a tamoxifen-inducible form of BLNK called BLNK-ER, down-regulation of the surface pre-BCR started around 12 hours after the induction of BLNK activity (data not shown). Assuming that this applies to the in vivo situation, IL-7R signaling would not be disrupted until 12 hours after the expression of pre-BCR on the surface of pre–B cells. In this model, BLNK itself would function as a negative regulator, or as an adaptor in a similar fashion as in the first model. In the former case, BLNK binding may alter JAK3 into an inactive conformation or disrupt the binding of positive regulators, such as SH2-Bβ for JAK2.43
We have demonstrated that constitutive activation of JAK3/STAT5 signaling is essential for the unlimited growth of our BLNK-pBLs; inhibition of the JAK3/STAT5 pathway by BLNK results in p27kip1 induction, followed by cell-cycle arrest. Since STAT5 is known to induce the expression of positive regulators for cell-cycle progression such as cyclin D and c-myc,44 it would be reasonable to think that the cell-cycle arrest induced by JAK3/STAT5 deactivation is primarily due to the reduction of such positive cell-cycle regulators. Therefore, it is surprising that BLNK-mediated deactivation of JAK3/STAT5 did not cause cell-cycle arrest in si-p27kip1 or si-Btk BKO84 cells where p27kip1 protein was not induced. Indeed, BLNK expression in BKO84 cells did not alter mRNA levels of cyclin D2, cyclin D3 and c-myc, or protein levels of cyclin D3 (data not shown). This indicates that active JAK3/STAT5 does not provide any signal-accelerating cell-cycle progression, but only the signal inhibiting p27kip1 expression in BLNK-pBLs, which underscores a previously unrecognized function of JAK3 and STAT5 in acting as a tumor promoter.
Taken together, we propose a multistep model of pre–B-cell leukemogenesis in BLNK−/− mice. In pre–B cells, signaling from both the pre-BCR and the IL-7R enables the cells to proliferate. According to our in vivo data,9 BLNK may positively regulate the initial proliferation of pre–B cells via the pre-BCR signaling pathway. After a few rounds of cell cycling and pre-BCR internalization, BLNK turns into a negative regulator of JAK3, as discussed; this normally functions as a negative feedback loop to inhibit pre–B-cell proliferation, together with down-regulation of IL-7R. The lack of BLNK may result in a reduction in the initial phase of pre-BCR–induced cell proliferation, but it also results in deregulated JAK3 activation through physiologic IL-7 stimulation, accelerated by up-regulated IL-7R expression.9 This results in the prolonged survival and accumulation of large pre–B cells, which is limited by the amount of physiologic IL-7. In these pre–B cells, where RAG1/2 expression is maintained,9,45 various genetic lesions may occur that enhance cell cycling. Among them, mutations that induce constitutive activation of IL-7R signaling, such as autocrine IL-7 expression, would be positively selected because of their ability to promote superior proliferation due to the lack of BLNK-mediated JAK3 inhibition. Additional mutations may stabilize the transformed state of the pre–B cells and endow malignant properties. Although a physiologic role for IL-7 in the B-cell development in humans is unclear, somatic gene modifications resulting in constitutive activation of IL-7R signaling and BLNK deficiency may cause pre–B-ALL in humans. To support this, 2 previous studies found constitutive STAT5 activity in the majority of ALL samples that they analyzed;46,47 some of these samples may well exhibit BLNK deficiency. In addition, we have found a few human pre–B-ALL cell lines in which BLNK expression is minimal and JAK3 is autonomously activated (data not shown).
Supplementary Material
Acknowledgments
We thank Dr T. Kitamura for pMX-IRES-EGFP, dnSTAT5a cDNA, and PLAT-E; and Dr S. Nishikawa for an αIL-7R hybridoma, ST2, and OP9.
This work is supported by grants to D.K. and R.G. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; to M.A.F. from the National Institutes of Health, the Cancer Research Institute; and the Leukemia & Lymphoma Society of America; and to D.K. from The Uehara Memorial Foundation and The Naito Foundation.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.N. and D.K. designed the research, analyzed data and wrote the paper; J.N., M.Y., K.H., and H.S. performed the research; K.B., M.K., R.G., and M.A.F. contributed vital reagents; and M.A.F. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daisuke Kitamura, Division of Molecular Biology, Research Institute for Biological Sciences, Tokyo University of Science, 2669 Yamazaki, Noda, Chiba 278-0022, Japan; e-mail: kitamura@rs.noda.tus.ac.jp.
References
- 1.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 2.Karasuyama H, Rolink A, Melchers F. Surrogate light chain in B cell development. Adv Immunol. 1996;63:1–41. doi: 10.1016/s0065-2776(08)60853-6. [DOI] [PubMed] [Google Scholar]
- 3.Osmond D, Rico-Vargas S, Valenzona H, et al. Apoptosis and macrophage-mediated cell deletion in the regulation of B lymphopoiesis in mouse bone marrow. Immunol Rev. 1994;142:209–230. doi: 10.1111/j.1600-065x.1994.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 4.Niiro H, Clark E. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 5.Kurosaki T, Tsukada S. BLNK: connecting Syk and Btk to calcium signals. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- 6.Minegishi Y, Rohrer J, Coustan-Smith E, et al. An essential role for BLNK in human B cell development. Science. 1999;286:1954–1957. doi: 10.1126/science.286.5446.1954. [DOI] [PubMed] [Google Scholar]
- 7.Jumaa H, Hendriks R, Reth M. B cell signaling and tumorigenesis. Annu Rev Immunol. 2005;23:415–445. doi: 10.1146/annurev.immunol.23.021704.115606. [DOI] [PubMed] [Google Scholar]
- 8.Flemming A, Brummer T, Reth M, Jumaa H. The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nat Immunol. 2003;4:38–43. doi: 10.1038/ni862. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi K, Yamamoto M, Nojima T, Goitsuka R, Kitamura D. Distinct signaling requirements for Dmu selection, IgH allelic exclusion, pre-B cell transition, and tumor suppression in B cell progenitors. Immunity. 2003;18:825–836. doi: 10.1016/s1074-7613(03)00142-0. [DOI] [PubMed] [Google Scholar]
- 10.Pui C, Robison L, Look A. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 11.Jumaa H, Bossaller L, Portugal K, et al. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature. 2003;423:452–456. doi: 10.1038/nature01608. [DOI] [PubMed] [Google Scholar]
- 12.Imai C, Ross M, Reid G, et al. Expression of the adaptor protein BLNK/SLP-65 in childhood acute lymphoblastic leukemia. Leukemia. 2004;18:922–925. doi: 10.1038/sj.leu.2403349. [DOI] [PubMed] [Google Scholar]
- 13.Sprangers M, Feldhahn N, Herzog S, et al. The SRC family kinase LYN redirects B cell receptor signaling in human SLP65-deficient B cell lymphoma cells. Oncogene. 2006;25:5056–5062. doi: 10.1038/sj.onc.1209510. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg R. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 15.Kersseboom R, Middendorp S, Dingjan G, et al. Bruton's tyrosine kinase cooperates with the B cell linker protein SLP-65 as a tumor suppressor in Pre-B cells. J Exp Med. 2003;198:91–98. doi: 10.1084/jem.20030615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan W, Alt F, Gerstein R, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 17.Hendriks R, Middendorp S. The pre-BCR checkpoint as a cell-autonomous proliferation switch. Trends Immunol. 2004;25:249–256. doi: 10.1016/j.it.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Xu S, Lee K, Huo J, Kurosaki T, Lam K. Combined deficiencies in Bruton tyrosine kinase and phospholipase Cgamma2 arrest B-cell development at a pre-BCR+ stage. Blood. 2007;109:3377–3384. doi: 10.1182/blood-2006-07-036418. [DOI] [PubMed] [Google Scholar]
- 19.Lu R, Medina K, Lancki D, Singh H. IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 2003;17:1703–1708. doi: 10.1101/gad.1104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolink A, Winkler T, Melchers F, Andersson J. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J Exp Med. 2000;191:23–32. doi: 10.1084/jem.191.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Namen A, Lupton S, Hjerrild K, et al. Stimulation of B cell progenitors by cloned murine interleukin-7. Nature: 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 22.Morrissey P, Conlon P, Charrier K, et al. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J Immunol. 1991;147:561–568. [PubMed] [Google Scholar]
- 23.Rich B, Campos-Torres J, Tepper R, Moreadith R, Leder P. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J Exp Med. 1993;177:305–316. doi: 10.1084/jem.177.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher G, Burdet C, LeMeur M, Haasner D, Gerber P, Ceredig R. Lymphoproliferative disorders in an IL-7 transgenic mouse line. Leukemia. 1993;7:S66–S68. [PubMed] [Google Scholar]
- 25.Tsuruyama T, Nakamura T, Jin G, Ozeki M, Yamada Y, Hiai H. Constitutive activation of Stat5a by retrovirus integration in early pre-B lymphomas of SL/Kh strain mice. Proc Natl Acad Sci U S A. 2002;99:8253–8258. doi: 10.1073/pnas.112202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall A, Fleming H, Wu G, Paige C. Modulation of the IL-7 dose-response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J Immunol. 1998;161:6038–6045. [PubMed] [Google Scholar]
- 27.Hess J, Werner A, Wirth T, Melchers F, Jack H, Winkler T. Induction of pre-B cell proliferation after de novo synthesis of the pre-B cell receptor. Proc Natl Acad Sci U S A. 2001;98:1745–1750. doi: 10.1073/pnas.041492098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erlandsson L, Licence S, Gaspal F, Lane P, Corcoran A, Martensson I. Both the pre-BCR and the IL-7Ralpha are essential for expansion at the pre-BII cell stage in vivo. Eur J Immunol. 2005;35:1969–1976. doi: 10.1002/eji.200425821. [DOI] [PubMed] [Google Scholar]
- 29.Cooper A, Sawai C, Sicinska E, et al. A unique function for cyclin D3 in early B cell development. Nat Immunol. 2006;7:489–497. doi: 10.1038/ni1324. [DOI] [PubMed] [Google Scholar]
- 30.Burchill M, Goetz C, Prlic M, et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171:5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- 31.Goetz CA, Harmon IR, O'Neil JJ, Burchill MA, Farrar MA. STAT5 activation underlies IL7 receptor-dependent B cell development. J Immunol. 2004;172:4770–4778. doi: 10.4049/jimmunol.172.8.4770. [DOI] [PubMed] [Google Scholar]
- 32.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci U S A. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi K, Nittono R, Okamoto N, et al. The B cell-restricted adaptor BASH is required for normal development and antigen receptor-mediated activation of B cells. Proc Natl Acad Sci U S A. 2000;97:2755–2760. doi: 10.1073/pnas.040575697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto M, Hayashi K, Nojima T, et al. BASH-novel PKC-Raf-1 pathway of pre-BCR signaling induces kappa gene rearrangement. Blood. 2006;108:2703–2711. doi: 10.1182/blood-2006-05-024968. [DOI] [PubMed] [Google Scholar]
- 35.Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui A, Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanada T, Yoshida T, Kinjyo I, et al. A mutant form of JAB/SOCS1 augments the cytokine-induced JAK/STAT pathway by accelerating degradation of wild-type JAB/CIS family proteins through the SOCS-box. J Biol Chem. 2001;276:40746–40754. doi: 10.1074/jbc.M106139200. [DOI] [PubMed] [Google Scholar]
- 37.Murata K, Kumagai H, Kawashima T, et al. Selective cytotoxic mechanism of GTP-14564, a novel tyrosine kinase inhibitor in leukemia cells expressing a constitutively active Fms-like tyrosine kinase 3 (FLT3). J Biol Chem. 2003;278:32892–32898. doi: 10.1074/jbc.M210405200. [DOI] [PubMed] [Google Scholar]
- 38.Li W, Jiang Q, Aleem E, Kaldis P, Khaled A, Durum S. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J Exp Med. 2006;203:573–582. doi: 10.1084/jem.20051520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen R, Wang D, McKay C, et al. Jak3 selectively regulates Bax and Bcl-2 expression to promote T-cell development. Mol Cell Biol. 2001;21:678–689. doi: 10.1128/MCB.21.2.678-689.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasuda T, Sanjo H, Pages G, et al. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28:499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 42.Engels N, Wollscheid B, Wienands J. Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-alpha. Eur J Immunol. 2001;31:2126–2134. doi: 10.1002/1521-4141(200107)31:7<2126::aid-immu2126>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 43.Kurzer J, Saharinen P, Silvennoinen O, Carter-Su C. Binding of SH2-B family members within a potential negative regulatory region maintains JAK2 in an active state. Mol Cell Biol. 2006;26:6381–6394. doi: 10.1128/MCB.00570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19:6613–6626. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 45.Sprangers M, Feldhahn N, Liedtke S, Jumaa H, Siebert R, Müsche M. SLP65 deficiency results in perpetual V(D)J recombinase activity in pre-B-lymphoblastic leukemia and B-cell lymphoma cells. Oncogene. 2006;25:5180–5186. doi: 10.1038/sj.onc.1209520. [DOI] [PubMed] [Google Scholar]
- 46.Weber-Nordt R, Egen C, Wehinger J, et al. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood. 1996;88:809–816. [PubMed] [Google Scholar]
- 47.Gouilleux-Gruart V, Gouilleux F, Desaint C, et al. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood. 1996;87:1692–1697. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.