Abstract
The immune response of cattle to nonliving vaccines derived from Brucella abortus rough strain 45/20 was studied. Vaccines contained trehalose dimycolate and a derivative of muramyl dipeptide. N-acetylmuramyl-L-alpha-aminobutyryl-D-isoglutamine. A factorial experiment was designed to test the effects of type of antigen, quantity of antigen, and quantity of mineral oil on the immune response to porin. Muramyl dipeptide was kept constant at 5 mg per dose, and 1 part of trehalose dimycolate was incorporated for two parts of dry matter. Over a 10-week period, blastogenesis responses to porin were largest in cattle immunized with outer membranes; the highest antibody titers to the porin-lipopolysaccharide complex were achieved by immunization with detergent-extracted outer membrane proteins. There was no advantage in the use of 25, rather than 5, mg of any of the antigens, but antibody responses were improved by increasing the quantity of oil from 0.6 to 1.8 ml per dose. In other animals, blastogenesis and antibody responses were sustained at high levels longer than 3 months after two vaccinations with outer membrane proteins. Intradermal injection of porin evoked inflammatory reactions histologically consistent with delayed-type hypersensitivity. Cross-reactions in cases of delayed-type hypersensitivity occurred with porin derived from a smooth strain of B. abortus but were less extensive than in the blastogenesis test. The magnitude of the delayed-type hypersensitivity and blastogenesis responses induced by vaccination exceeded those observed after natural or experimental infections. No ill effects were observed after vaccination. These findings provide a basis for the use of trehalose dimycolate and muramyl dipeptide adjuvants in evaluating nonviable vaccines for bovine brucellosis.
Full text
PDF



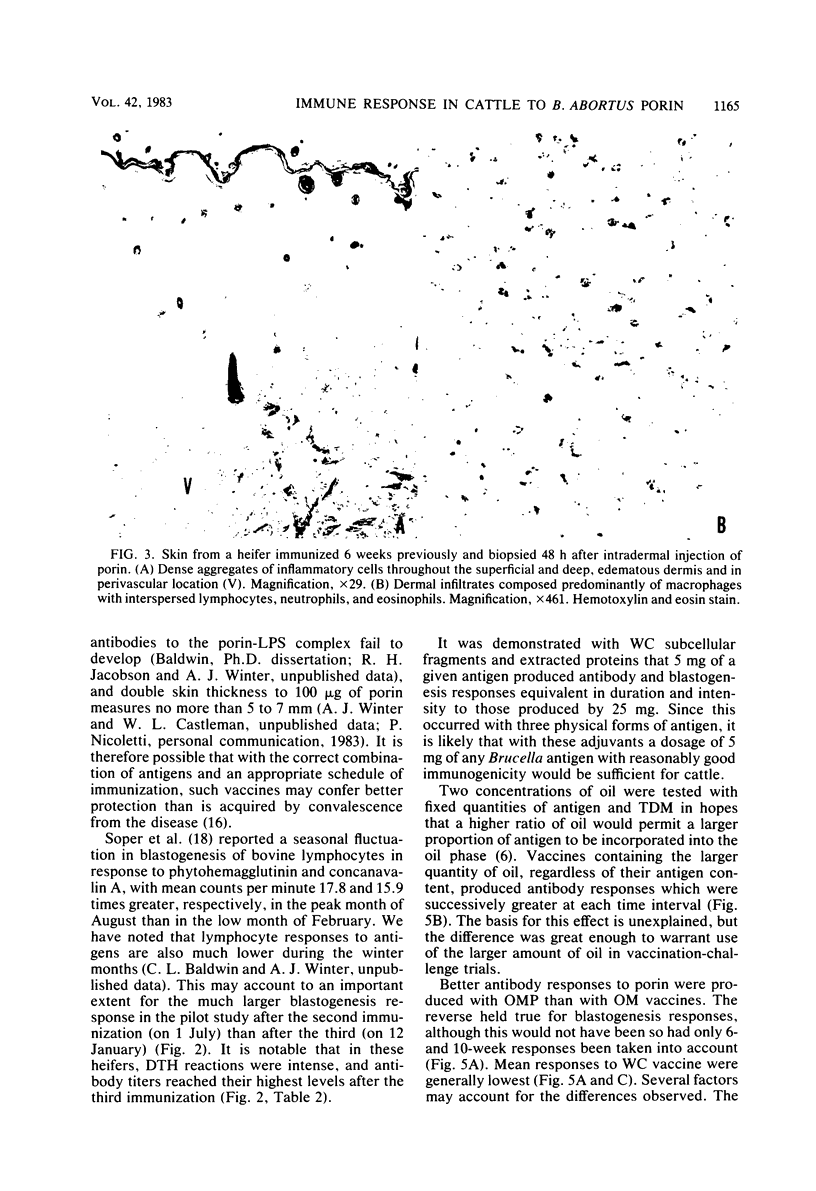
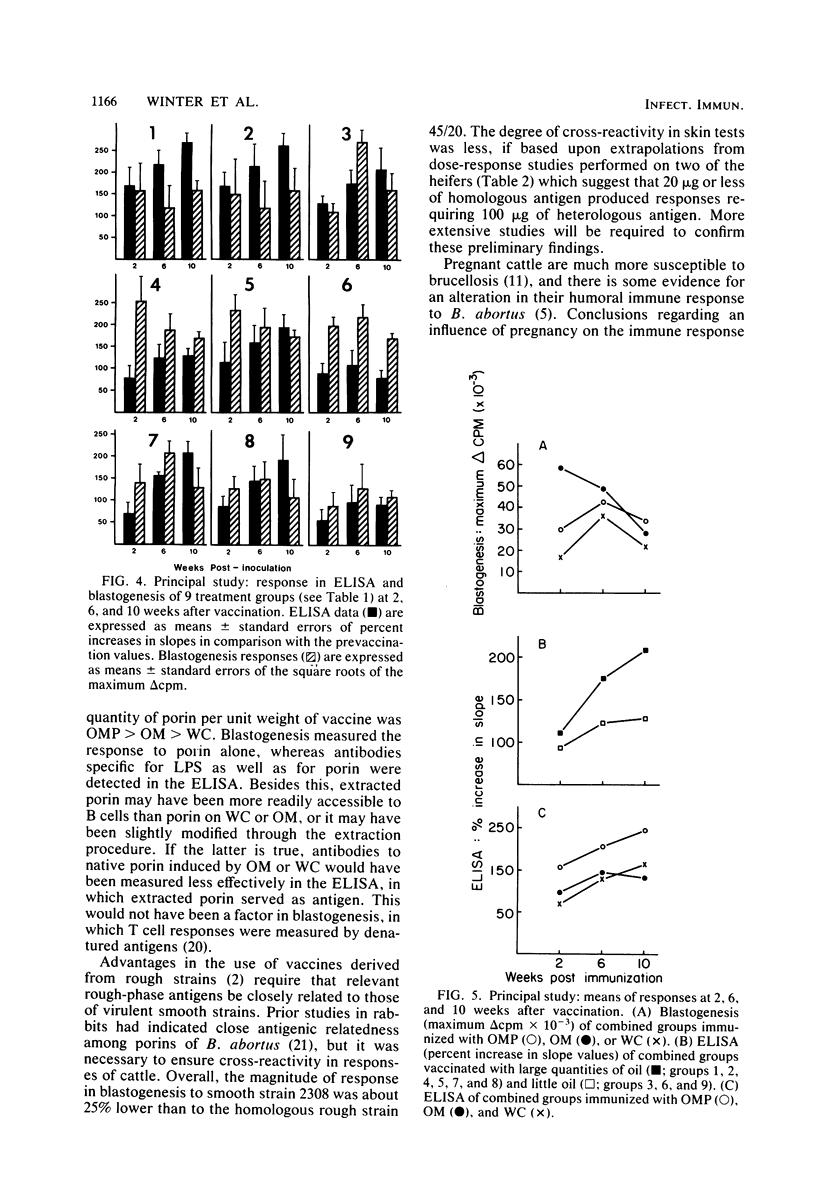

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Petit J. F., Lefrancier P., Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981 Dec 4;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- Azuma I., Ribi E. E., Meyer T. J., Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst. 1974 Jan;52(1):95–101. doi: 10.1093/jnci/52.1.95. [DOI] [PubMed] [Google Scholar]
- BIENVENU R. J., Jr, YOUNG V. F. The relationships of pregnancy hormones to brucellacidal activity in bovine serum. Am J Vet Res. 1962 Sep;23:1027–1030. [PubMed] [Google Scholar]
- Barlough J. E., Jacobson R. H., Downing D. R., Marcella K. L., Lynch T. J., Scott F. W. Evaluation of a computer-assisted, kinetics-based enzyme-linked immunosorbent assay for detection of coronavirus antibodies in cats. J Clin Microbiol. 1983 Feb;17(2):202–217. doi: 10.1128/jcm.17.2.202-217.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Yamamoto K. I., Ribi E. Delayed hypersensitivity and granulomatous response after immunization with protein antigens associated with a mycobacterial glycolipid and oil droplets. J Immunol. 1976 Feb;116(2):482–488. [PubMed] [Google Scholar]
- Lederer E. Synthetic immunostimulants derived from the bacterial cell wall. J Med Chem. 1980 Aug;23(8):819–825. doi: 10.1021/jm00182a001. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madraso E. D., Cheers C. Polyadenylic acid-polyuridylic acid (poly A : U) and experimental murine brucellosis. II. Macrophages as target cells of poly A : U in experimental brucellosis. Immunology. 1978 Jul;35(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982 Nov;152(2):822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti P. The epidemiology of bovine brucellosis. Adv Vet Sci Comp Med. 1980;24:69–98. [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon P. Resistance against a subcutaneous Brucella challenge of mice immunized with living or dead Brucella or by transfer of immune serum. Ann Immunol (Paris) 1977 Nov-Dec;128(6):1025–1037. [PubMed] [Google Scholar]
- Pavlov H., Hogarth M., McKenzie I. F., Cheers C. In vivo and in vitro effects of monoclonal antibody to Ly antigens on immunity to infection. Cell Immunol. 1982 Jul 15;71(1):127–138. doi: 10.1016/0008-8749(82)90502-0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Soper F. F., Muscoplat C. C., Johnson D. W. In vitro stimulation of bovine peripheral blood lymphocytes: analysis of variation of lymphocyte blastogenic response in normal dairy cattle. Am J Vet Res. 1978 Jun;39(6):1039–1042. [PubMed] [Google Scholar]
- Sulitzeanu D. Mechanism of immunity against brucella. Nature. 1965 Mar 13;205(976):1086–1088. doi: 10.1038/2051086a0. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Webster A. S. Can the lumpy distribution of galaxies be detected by X-ray observations? Nature. 1972 Jul 7;238(5358):20–24. doi: 10.1038/238020a0. [DOI] [PubMed] [Google Scholar]
- Woodard L. F., Renshaw H. W., Burger D., McCain C. S., Wilson R. B. Cell-mediated immune responses of neonatal calves and adult cattle following inoculation with PPD of Mycobacterium bovis associated with a mycobacterial immunopotentiating glycolipid and oil droplets. Am J Vet Res. 1979 May;40(5):636–644. [PubMed] [Google Scholar]
- Woodard L. F., Toone N. M., McLaughlin C. A. Comparison of muramyl dipeptide, trehalose dimycolate, and dimethyl dioctadecyl ammonium bromide as adjuvants in Brucella abortus 45/20 vaccines. Infect Immun. 1980 Nov;30(2):409–412. doi: 10.1128/iai.30.2.409-412.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



