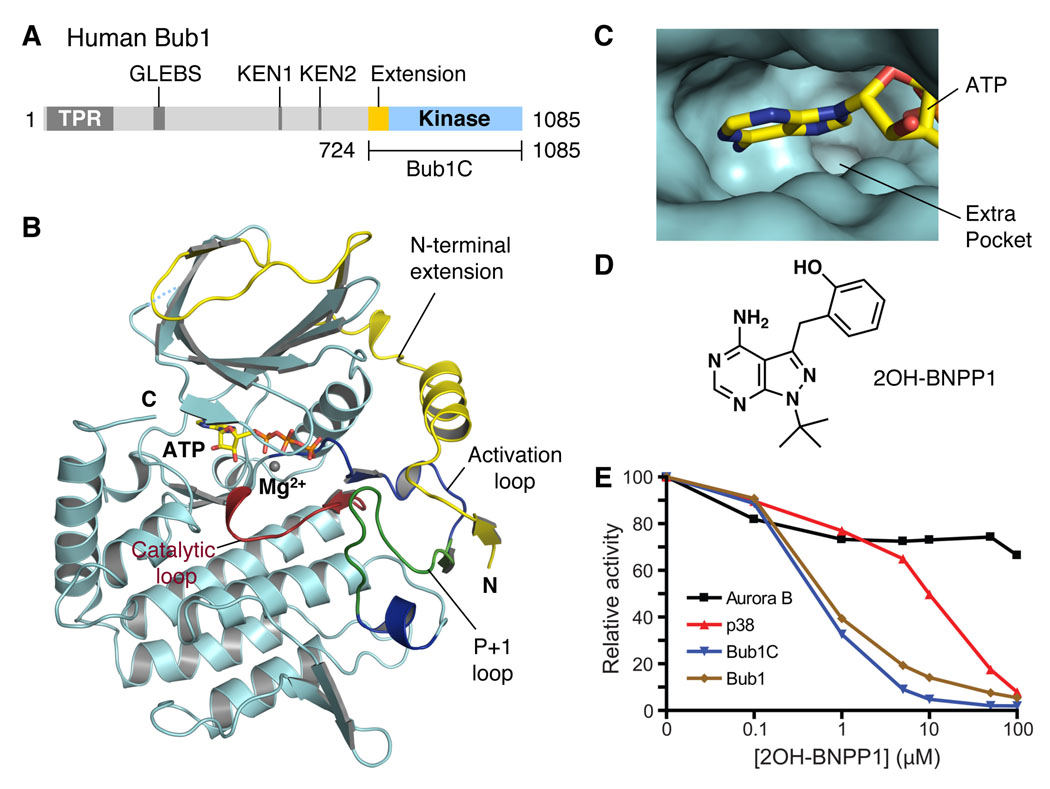
Figure 1. Structure and Inhibitor of the Extended Kinase Domain of Bub1.
(A) Domain architecture of human Bub1. TPR, tetratricopeptide repeat; GLEBS, Gle2-binding sequence; KEN, lysine-glutamate-asparagine.
(B) Ribbon drawing of the crystal structure of the extended kinase domain of Bub1. The N-terminal extension is colored yellow. The substrate-binding P+1 loop is shown in green while the rest of the activation segment is in blue. The catalytic loop is shown in red. ATP is shown as sticks. The Mg2+ ion is shown as a gray sphere. The N- and C-termini are indicated. All structure figures were generated with PyMOL (http://pymol.sourceforge.net/).
(C) The ATP-binding site of Bub1. ATP is shown as sticks. The extra pocket is indicated.
(D) The chemical structure of 2OH-BNPP1.
(E) Determination of the IC50 values of 2OH-BNPP1 against Aurora B, p38, Bub1C, and Bub1.