Abstract
The antioxidant activity of four honey samples from different floral sources (Acacia, Coriander, Sider and Palm) were evaluated with three different assays; DPPH free radical scavenging assay, superoxide anion generated in xanthine–xanthine oxidase (XOD) system and low density lipoprotein (LDL) peroxidation assay. The dark Palm and Sider honeys had the highest antioxidant activity in the DPPH assay. But all the honey samples exhibited more or less the same highly significant antioxidant activity within the concentration of 1mg honey/1 ml in XOD system and LDL peroxidation assays. The chemical composition of these samples was investigated by GC/MS and HPLC analysis, 11 compounds being new to honey. The GC/MS revealed the presence of 90 compounds, mainly aliphatic acids (37 compounds), which represent 54.73, 8.72, 22.87 and 64.10% and phenolic acids (15 compound) 2.3, 1.02, 2.07 and 11.68% for Acacia, Coriander, Sider and Palm honeys. In HPLC analysis, 19 flavonoids were identified. Coriander and Sider honeys were characterized by the presence of large amounts of flavonoids.
Keywords: Antioxidant, GC/MS, Honey, HPLC, LDL peroxidation
Introduction
Free radicals and reactive oxygen species (ROS) have been implicated in contributing to aging and many disease states including cancer and atherosclerosis. Antioxidants are compounds that can delay or inhibit the oxidation of lipids or other molecules by inhibiting the initiation or propagation of oxidizing chain reactions (1). Many synthetic antioxidant components have shown toxic and/or mutagenic effects, which directed most of the attention on the naturally occurring antioxidants. Their use has mainly centered on prevention, and the maintenance of health (2–5).
The oxidative modification hypothesis of atherosclerosis predicts that low-density lipoprotein (LDL) oxidation is an early event in atherosclerosis (6). Therefore, inhibition of LDL oxidation might be an important step in preventing atherogensis (7,8).
Humans protect themselves from (ROS), in part, by absorbing dietary antioxidants. Thus, increasing the body's antioxidant content may help protect against cellular damage and the development of chronic diseases. Research indicates that honey contains numerous phenolic and non-phenolic antioxidants (9), the amount and type of which depends largely upon the floral source of the honey. Darker honeys are generally higher in antioxidant content than lighter honeys and have been shown to be similar in antioxidant capacity to many fruits and vegetables on a dry weight basis (9–12). Honey has a great potential to serve as a natural food antioxidant. The antioxidant activity of honey, however, varies greatly depending on the honey floral source (10,13).
A strong correlation between antioxidant activity of honeys and total phenolic content was previously demonstrated (10). In several studies on European honeys, Ferreres and co-workers have shown that honeys have a rich phenolic profile consisting of benzoic acids and their esters, cinnamic acids and their esters and flavonoid aglycones (14–18). In general, the antioxidant capacity of honey appeared to be a result of the combined activity of a wide range of compounds including phenolics, peptides, organic acids, enzymes and possibly other minor components. The phenolic compounds contributed significantly to the antioxidant capacity of honey, but were not solely responsible for it (9). However, little information is available on the phenolic and non-phenolic profiles of honeys from floral sources common in Arabic region. Characterization of the phenolics and other components in honey that might be responsible for its antioxidant effects is essential to improve our knowledge about honey as a source of antioxidants.
The objective of this study was, therefore, to identify and quantify the chemical composition of four honey samples from different floral sources by GC/MS and HPLC and to find out (for the first time) the highly effective antioxidant one which could protect the human LDL against copper-induced oxidation in vitro—a study that provides a primary evidence—for further in vivo studies.
Materials and Methods
Reagents and Honey
All reagents are of analytical purity grade. Distilled water was used for all dilution steps. Acacia, Coriander and Palm honeys were collected as market samples (From an authorized apiary farm) of Egyptian origin, while Sider honey was kindly provided by El-Yahia Company, Saudi Arabia (2004, flowering season). All of these honeys are vended as ‘monofloral’, meaning that the honey must derive from at least 55% of pollen from a single floral source according to Louveaux et al. (19).
Extraction of Honey for GC/MS
Fifty grams of each honey sample were extracted with diethyl ether (20), and concentrated by evaporation under vacuum at 40°C. Five milligrams were of the ether extract was dissolved in 0.05 ml pyridine + 0.1 ml BSTFA [N,O-bis (trimethylsilyl) trifluoro-acetamide (BSTFA), from Sigma] and heated for 30 min at 60°C and injected in the GC/MS (21).
Flavonoid Extraction for HPLC
Two hundred grams of each honey sample were passed through a column (25 × 2 cm) of Amberlite XAD-2 (Supelco; pore size 9 nm, particle size 0.3–1.2 mm). The phenolic fraction was dissolved in methanol and filtered through 0.45-μm filter before direct HPLC analysis (16).
Determination of DPPH Free Radical Scavenging Activity
The DPPH (1,1-diphenyle-2-picryl-hydrazyl) radical scavenging activity was determined according to the method of Matsushige (22). The absorbance was measured at 520 nm. Honey samples were dissolved in distilled water and 6 μM DPPH was dissolved in methanol. Mean of three measurements of each sample was calculated.
Determination of Superoxide Anion Radical Scavenging Activity
The superoxide anion radical scavenging activity by generating superoxide anion free radical in xanthine–xanthine oxidase (X–XOD) system was measured (22). The color obtained was measured at 560 nm. Mean of three measurements of each sample were calculated.
Measurement of Copper-Induced Low Density Lipoprotein (LDL) oxidation In-Vitro
Isolation of LDL
LDL was isolated according to the method of Gugliucci and Menini (23). LDL (1.019–1.055 g/ml) was separated by sequential ultra-centrifugation using TL-100 Ultracentrifuge (Beckman, USA) from plasma. LDL then extensively dialyzed against phosphate-buffered saline (PBS), pH 7.2, containing 0.01% EDTA at 4°C. Samples were stored at 4°C in the dark and used within 24 h. Protein content was determined according to Lowry's (determination of protein kit) method (24).
Thiobarbituric Acid Reactive Substances (TBARS) Assay
LDL was oxidized using 5 μM/ml CuSO4 (25). Oxidation of LDL was monitored in the presence or absence of honey sample by measuring the thiobarbituric acid reactive substances (TBARS). The absorbance was measured at 534 nm using UV Spectrophotometer [UNICAM UV300], malondialdehyde-bis-(dimethylacetal) which yields malondialdehyde (MDA) by acid treatment, was used as a standard.
GC/MS Analysis
A finnigan MAT SSQ 7000 mass spectrometer was coupled with a Varian 3400 gas chromatograph. DB-5 column, 30 m × 0.32 mm (internal diameter), was employed with helium as carrier gas and the temperature programmed from 40°C to 260°C at 5°C/min (3 min initial hold, 10 min final hold). The mass spectra were recorded in electron ionization (EI) mode at 70 eV, ion source temperature 150°C. The scan repetition rate was 0.5 s.
Identification of Compounds
Peaks were identified by computer search of user-generated reference libraries, incorporating mass spectra. Peaks were examined by single-ion chromatographic reconstruction to confirm their homogeneity; mixed peaks were resolved by computer program aimed at resolving the mass spectral data of one compound from overlapping mass spectra of another.
HPLC Analysis of Honey Flavonoids
The HPLC analysis was achieved with Agilent 1100 series liquid chromatograph with UV detector and an auto-sampler. The column used was a Lichrochart RP-18 (Merck, Darmstadt, Germany; 25 × 0.4 cm, 5-μm particle size). Elution was with water: formic acid (19 : 1 v:v; solvent A) and acetonitrile (solvent B), and the flow rate was 1 ml/min. Gradient elution started with 20% B, reaches 25% B at 25 min and 30% B at 35 min, and then the system became isocratic until 50 min, reaches 50% B at 60 min and 70% B at 67 min. The flavonoids were detected with UV detector and the chromatograms were recorded at 340 and 290 nm.
Flavonoid Identification and Quantification
The different flavonoids were identified by chromatographic comparisons with authentic flavonoids, some of them are commercial and most were kindly provided by Prof. Wollenweber, (Institut of Botanik Schittspahnstr. TU Darmstadt, Germany). The flavanones were detected at 290 nm and the flavones at 340 nm. Flavonoid identification was carried out by direct HPLC comparison of authentic flavonoids and was based on co-chromatography in 290 and 340 nm. Response factors for the authentic flavonoids and the concentration of flavonoids in each honey sample were calculated (26,27).
Statistical Analysis
Data were analyzed statistically using Student's t-test showing mean + SD. Data were compared using one way. Statistical significance was accepted at P < 0.01 (28).
Results
The DPPH Free Radical Scavenging Activity
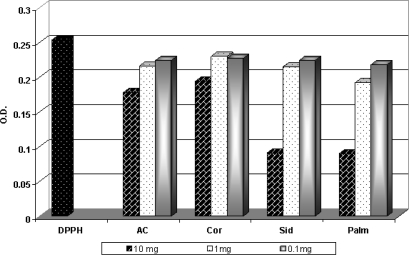
The highly antioxidant activity in DPPH scavenging assay was ∼64.7% in conc.10 mg honey/ml. The concentration (1 mg honey/ml) showed a lower activity ranged from 24.11% to 14.11%, while the lowest activity (13.83–9.00%) appeared with the honey concentration (0.1 mg honey/ml). It is clear that the darker Palm and Sider honeys had the highest antioxidant activity (64%) in DPPH scavenging assay while the lowest antioxidant activity was observed in the lighter Acacia and Coriander honeys (30.5 and 23.9%, respectively) at the concentration 10 mg honey/ml (Fig. 1).
Figure 1.
The free radical scavenging activity of honey samples against DPPH radical.
Scavenging Ability for Superoxide Anion Radical
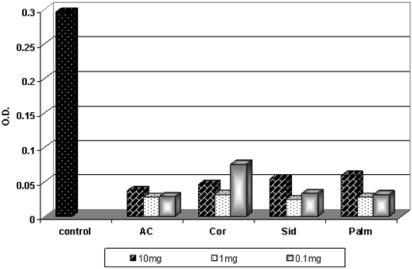
The free radical scavenging activity on superoxide anion radical (O−2) generated by an enzymatic method was evaluated. The results are shown in Fig. 2. The results demonstrated that all the honey samples (the darker and the lighter), and in all the concentrations, were highly effective against O−2. All the honey samples exhibited more or less the same high antioxidant activity within the concentration 1 mg honey/1 ml, which ranged from 91.58% to 89.22%. In contrast, the concentration (10 mg honey/ml) showed a lower activity than that of 1 mg honey/ml which ranged from 87.2% to 79.80%.
Figure 2.
The free radical scavenging activity of honey samples in Xanthin–XOD system.
Susceptibility of LDL to Cu2+-induced Oxidation
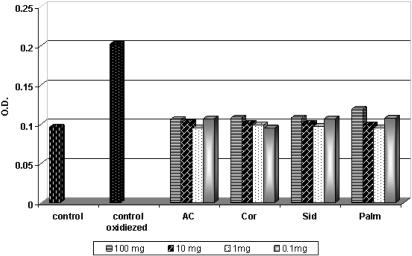
Pre-incubation of LDL with honey samples resulted in significant inhibition of TBARS accumulation. From the data shown in Fig. 3, clearly that in the LDL peroxidation assay, all the honey types under this experiment exhibited more or less the same high antioxidant activity within the concentration of 1 mg honey/1 ml (0.095–0.099, i.e. it has the same result of the control), while the concentrations (100 mg and 10 mg honey/ml) showed lower activity. However, the results clarified that all honey samples (the darker and the lighter), and in all concentrations, were highly effective against LDL peroxidation, and the results are more or less near to the control result. The TBARS, as an index of lipid peroxidation, were undetectable in control LDL, slightly rising only after 3 h of incubation. Incubation with the oxidant resulted in a marked elevation of TBARS. After 24 h of incubation in the presence of the oxidant, TBARS level did not further increase significantly [data not shown].
Figure 3.
Inhibitory effect of honey samples on cupper-induced LDL oxidation.
Chemical Composition of Honey
GC/MS Analysis
Investigations of ether extracts of the four honey samples by GC/MS revealed the presence of 90 compounds, nine of which are new to honey. The main compounds are aliphatic acids (37 compounds), which represent 54.73, 8.72, 22.87 and 64.10% for Acacia, Coriander, Sider and Palm honeys (Table 1). The presence of 10 aliphatic dioic acids represents 5.91, 0.16, 1.17 and 37.66% for Acacia, Coriander, Sider and Palm honeys. Succinic acid as a dioic acid was only present in Palm honey with a high concentration (28.72%), 3-hydroxy-sebacic acid was only present in Acacia honey. Decandioic acid was present with a large amount in Palm and Acacia honeys. Palm honey contained most of these dioic acids, while Coriander honey contained a little number with very small amounts of them. Methyl butandioic acid was the only dioic acid shared in all honey samples. Octandioic and nonandioic acids were present in Acacia honey in high amounts (0.66 and 0.94%), they were also present in Coriander and Sider honeys.
Table 1.
Chemical composition assessed by GC/MS of ether extracts of honey samples
| Compound | Acacia | Coriander | Sider | Palm |
|---|---|---|---|---|
| Aliphatic acids | % TIC a | |||
| Hydroxyacetic acid | – | 0.03 | 0.06 | 0.17 |
| 2-Hydroxypropanoic acid | 18.70 | 0.21 | 0.04 | 0.56 |
| 3-Hydroxypropanoic acid | 0.40 | 0.07 | 0.01 | 0.13 |
| 2,3-Dihydroxypropanoic acid | 0.32 | 0.04 | 2.04 | 0.82 |
| 2-Oxo-3-hydroxypropanoic acid | – | – | 0.04 | – |
| Lactic acid dimmer | – | – | – | 0.05 |
| 2-Methyl-2-hydroxybutanoic acid | 0.05 | – | – | – |
| 3-Methyl-3-hydroxybutanoic acid | 0.04 | – | – | – |
| 5-Hydroxy-n-valeric acid | 8.27 | 3.62 | 0.21 | – |
| 4-Oxo-pentanoic acid | 0.11 | – | – | – |
| Pentanoic acid-2-deoxy-3,5-dihydroxy | – | 0.30 | – | – |
| Pentanoic acid-5-deoxy-2,3-dihydroxy-γ-lactone | – | 0.05 | – | – |
| Pentanoic acid-5-deoxy-2,3-dihydroxy-γ-lactone (isomer) | – | 0.30 | – | – |
| 2,3,4,5-Tetrahydroxypentanoic acid-1,4-lactone | – | – | 2.07 | – |
| 2,3,4,5-Tetrahydroxypentanoic acid-1,4-lactone(isomer) | – | – | 0.09 | – |
| Succinic acid b | – | – | – | 28.72 |
| Malic acid (hydroxyl-succinic acid)b | 0.30 | 1.16 | ||
| 2-butenedioic acid (E) b | 0.07 | – | – | 1.25 |
| Methyl butandioic acid b | 0.14 | 0.02 | 0.01 | 0.30 |
| 2-Hexenoic acid | 10.50 | – | 0.08 | – |
| Pentanedioic acid b | – | 0.01 | – | 0.37 |
| 7-Methyl- pentanedioic acid b | – | – | – | 0.12 |
| 3-Hydroxy caproic acid | – | 0.30 | 0.15 | – |
| 7-Hydroxy-octanoic acid | – | – | 0.03 | 0.20 |
| Octandioic acid b | 0.66 | 0.04 | 0.13 | – |
| 2,3,5-Trihydroxyxylonic acid-γ-lactone | – | 0.04 | – | – |
| Nonandioic acid(azelic acid) b | 0.94 | 0.09 | 0.35 | – |
| Decandioic acid(sebacic acid) b | 3.29 | – | 0.68 | 5.74 |
| 3-Hydroxy-sebacic acid b | 0.51 | – | – | – |
| Tetradecanoic acid | 0.17 | 0.06 | 0.20 | – |
| Pentadecanoic acid | – | – | 0.19 | – |
| Palmitic acid | 4.75 | 0.9 | 3.39 | 1.50 |
| Oleic acid | 5.41 | 1.53 | 3.34 | – |
| Stearic acid | – | 0.79 | 1.04 | – |
| Ecosanoic acid | 0.09 | 0.18 | – | 0.14 |
| Docosanoic acid | – | 0.14 | – | – |
| Aromatic acids | ||||
| Benzoic acid | 0.07 | – | – | – |
| 2- Aminobenzoic acid c | 0.03 | |||
| 3,4-Dimethoxybenzoic acid | – | 0.11 | – | – |
| 4-Hydroxy benzoic acid | 0.32 | 0.57 | 1.67 | 4.15 |
| Vanillic acid | 0.36 | 0.14 | 0.27 | 3.05 |
| 3,4-Dihydroxybenzoic acid | – | 0.04 | 0.07 | – |
| 4-Hydroxybenzene propanoic acid | 0.05 | – | – | 0.30 |
| 2-Furancarboxylic acid | 0.12 | 0.02 | – | – |
| 2-Furancarboxylic acid-5-hydroxymethyl | 1.14 | 0.07 | – | 0.44 |
| Furyl acrylic acid c | – | – | 0.03 | – |
| Cinnamic acid | 0.09 | – | – | – |
| p-Hydroxydihydro-cinnamic acid | – | – | 0.56 | – |
| 3,4- Dimethoxy-cinnamic acid | – | – | – | 3.00 |
| 2,5- Dimethoxy-cinnamic acid | – | – | – | 0.17 |
| Cis- p-Coumaric acid | 0.15 | 0.05 | 0.07 | 0.21 |
| Caffeic acid | – | – | – | 0.36 |
| Others | ||||
| 1-Methyl pentanol | – | – | – | 0.06 |
| 2,3-Butane diol | 0.52 | – | – | – |
| 2,3-Butane diol(isomer) | 0.8 | – | – | – |
| 3-Methyl-1,3-dihydroxy butane | 0.01 | – | – | – |
| Glycerol | – | – | – | 0.8 |
| Phosphoric acid | – | 0.05 | 0.02 | 0.04 |
| 3-Hydroxypyridine c | – | – | – | 0.03 |
| Picolinic acid(pyridine carboxylic acid) c | – | – | – | 0.03 |
| 1,2- cyclohexane dicaboxylic acid | – | 0.09 | – | 0.89 |
| 1,4-Dihydroxy benzene | – | 0.07 | – | 1.06 |
| 2,3-Dimethoxy benzaldehyde | – | 0.02 | – | – |
| 4-Hydroxy phenyl ethanol | – | 0.02 | – | 0.11 |
| Vanillyl alcohol | – | 0.04 | – | – |
| 1,2-Benzenediol-3,5-bis(1,1-dimethylethyl) | – | – | 0.20 | – |
| 2,4-bis(dimethyl benzyl)-6-t- butyl phenol | 0.52 | – | – | – |
| 2(3H)-Furanne-dihydro-3,4-dihydroxy-(trans) | – | 0.08 | – | 0.55 |
| 4H-pyran-4-one-5-hydroxy-2-hydroxymethyl | – | 0.04 | – | 0.39 |
| 4H-pyran-4-one-5-hydroxy-2-hydroxymethyl (isomer) | – | – | – | 1.11 |
| Octadecanyl glycerol ether | 0.06 | 1.4 | – | – |
| Eicosanyl glycerol ether | 0.14 | 1.6 | – | – |
aThe ion current generated depends on the characteristics of the compound concerned and it is not a true quantitation.
bDioic acid.
cFor the first time in honey.
Acacia honey was characterized by the presence of high percentage of 2-hydroxypropanoic acid; 5-hydroxy-n-valeric acid and 2-hexenoic acid; benzoic acid; cinnamic acids; 2,3-butanediol; 2,3-butanediol(isomer); 3-methyl-1,3-dihydroxybutane; 2,4-bis(dimethyl benzyl)-6-t-butyl phenol and 2-Methyl-3-hydroxypropanoic acid.
Coriander honey was the only sample that showed 3,4-dimethoxybenzoic acid and 3,4-dimethoxybenzene acetic acid, 2,3-dimethoxybenzaldehyde and vanillyl alcohol.
Sider honey was characterized by the presence of 2-oxo-3-hydroxypropanoic acid; 2,3,4,5-tetrahydroxypentanoic acid-1,4-lactone; p-hydroxy-dihydrocinnamic acid and 1,2-benzenediol-3,5-bis(1,1-dimethylethyl). 2-Aminobenzoic acid and furyl acrylic acids were present for the first time.
Palm honey was characterized by having a high significant amount of succinic acid (28.72%). Also it contained 3,4- dimethoxy-cinnamic acid; 2,5- dimethoxy-cinnamic acid; caffeic acid; 1-methyl pentanol and 4H-pyran-4-one-5-hydroxy-2-hydroxymethyl isomer. 3-hydroxypyridine and picolinic acid (pyridine carboxylic acid) were present for the first time.
Nine fatty acid esters were present in Coriander honey: monoethylsuccinate, ethyl palmitate, ethyloleate, ethylstearate, 12-hydroxy stearic acid methyl ester, palmitic acid decyl ester, oleic acid octyl ester, docosanoic acid ethyl ester and tetracosanoic acid ethyl ester (0.05, 0.18, 0.7, 0.03, 0.08, 0.22, 0.38, 0.08 and 0.15%). Methyloleate was found only in Acacia honey (0.37%), while ethyloleate and docosanoic acid ethyl ester were present in Sider honey (0.13 and 0.04%). Palm honey did not contain any fatty acid esters (data not shown in Table 1).
It is the first time to identify the diterpene, dehydroabietic acid in honey, in Acacia, Coriander and Sider honeys (0.8, 0.08 and 0.04%—data not shown in Table 1). Five new dihydroxy-methyl anthraquinones were detected in Acacia, Coriander and Palm honeys (1.53, 1.37 and 1.03%—data not shown in Table 1). Sider honey did not contain any anthraquinones.
HPLC Analysis
The flavonoids present in four honey samples were studied by HPLC analysis. A total of 23 flavonoids were detected in the four honey samples, from which 19 were completely identified. The difference in flavonoid composition between the four honey samples is clear in Table 2. Coriander honey has the highest content of myricetin, eriodictyol, naringenin, 8-methoxy kaempferol, apigenin, kaempferol, quercetin and quercetin -3,3′-dimethylether, Liquiriteginin, luteolin and quercetin -7-methylether were present only in Coriander honey. Pinobankasin and formonontin were present only in Sider honey. Coriander and Sider honeys were characterized by the presence of large amounts of flavonoids. Acacia and Palm honeys were characterized by the presence of lesser amounts of flavonoids. Liquiriteginin and formonontin were identified for first time in honey.
Table 2.
Flavonoids detected in honey samples by HPLC technique (μg/100 g honey)
| No | Name | Structure | Origin | Acacia | Coriander | Sider | Palm |
|---|---|---|---|---|---|---|---|
| 1 | Major unknown (Mμ) | – | – | (Mu) | – | – | |
| 2 | Major unknown(Mμ) | – | – | (Mu) | – | – | |
| 3 | Myricetin | 3,5,7,3′,4′,5′-Hexahydroxyflavone | Pollen-nectar | – | 188.15 | – | 4.37 |
| 4 | Liquiriteginin | 7,4′-Dihydroxyflavanone | Pollen-nectar | – | 75.23 | – | – |
| 5 | Eriodictyol | 5,7,3′,4′-Tetrahydroxyflavanone | Pollen-nectar | – | 170.06 | 80.23 | 21.15 |
| 6 | Luteolin | 5,7,3′,4′-Tetrahydroxyflavone | Pollen-nectar | – | 22.22 | – | – |
| 7 | Quercetin | 3,5,7,3′,4′-Pentahydroxyflavone | Pollen-nectar | 17.58 | 28.47 | – | 3.41 |
| 8 | Naringenin | 5,7,4′-Trihydroxyflavanone | Pollen-nectar | – | 154.78 | 90.08 | 8.01 |
| 9 | Pinobankasin | 3,5,7-Trihydroxyflavanone | Propolis | – | – | 24.65 | – |
| 10 | Quercetin-3-methylether | 5,7,3′,4′-Tetrahydroxy-3-methoxyflavone | Propolis | – | 9.87 | 18.32 | 0.90 |
| 11 | Genistein | 5,7,4′-Trihydroxyisoflavone | 1.04 | – | – | 9.88 | |
| 12 | Hesperetin | 5,7,3′-Trihydroxy-4′-methoxyflavanone | Pollen-nectar | – | 21.22 | 159.33 | – |
| 13 | 8-Methoxykaempferol | 3,5,7,4′-Tetrahydroxy-8-methoxyflavone | Pollen-nectar | 1.63 | 23.67 | – | 0.20 |
| 14 | Apigenin | 5,7,4′-Trihydroxyflavone | Pollen-nectar | 0.12 | 19.81 | – | 0.20 |
| 15 | Major unknown (Mμ) | – | Pollen-nectar | – | – | – | Mu |
| 16 | Kaempferol | 3,5,7,4′- Tetrahydroxyflavone | Pollen-nectar | 2.36 | 27.80 | 20.07 | 4.36 |
| 17 | Luteolin-3′-methylether | 5,7,4′-Trihydroxy-3′-methoxyflavone | Pollen-nectar | – | 34.29 | 43.27 | – |
| 18 | Kaempferol-3-methylether | 5,7,4′-Trihydroxy-3-methoxyflavone | Propolis | – | 37.34 | 38.14 | 14.62 |
| 19 | Quercetin-3,3′-dimethylether | 5,7,4′-Trihydroxy-3,3′-dimethoxyflavone | Propolis | – | 14.26 | 12.36 | 10.38 |
| 20 | Formonontin | 7-Hydroxy-4′-methoxyisoflavone | – | – | 12.43 | – | |
| 21 | Quercetin-7-methylether | 3,5,3′,4′-Tetrahydroxy-7-methoxyflavone | Propolis | – | 3.08 | – | – |
| 22 | Major unknown (Mμ) | – | Mu | – | – | – | |
| 23 | Prunetin | 5,4′-Dihydroxy-7-methoxyisoflavone | 10.16 | – | – | – | |
| Total flavonoids | 32.89 | 770.25 | 498.88 | 77.28 |
Discussion
Increasing the body's antioxidant content may help protect against cellular damage and the development of chronic diseases. Research indicates that honey contains numerous phenolic and non-phenolic antioxidants (9). LDL peroxidation is considered to be essential in the pathogenesis of atherosclerosis (6). Compounds with antioxidant activity could have some beneficial effects in preventing atherosclerosis (29). In this study, we set out to demonstrate the antioxidant properties of four different honeys (Acacia, Coriander, Sider and Palm honeys) employing three different assays: The DPPH radical scavenging assay, superoxide generated in X–XOD system and (for the first time) the LDL peroxidation assay.
In the DPPH radical system, antioxidant directly reacts with DPPH radical. The dark Palm and Sider honeys had the highest antioxidant activity in DPPH scavenging assay while low antioxidant activity was observed in the light Acacia and Coriander honeys at the concentration (10 mg honey/ml). This result is consistent with the study by Inoue et al. (30) who showed that DPPH radical scavenging activity was significantly different among honeys; with the dark buckwheat and manuka honeys having significantly higher scavenging activity than acacia honey. In addition, Gheldof and Engeseth (10) found that darker honeys (e.g. buckwheat) are generally higher in antioxidant content than lighter honeys and have been shown to be similar in antioxidant capacity to many fruits and vegetables on a dry weight basis.
In X–XOD system, superoxide anion radical is enzymatically generated. The harmful effect of superoxide is reduced by superoxide dismutase enzyme (SOD) present in the animal body, honey also showed similar activities to that of the SOD enzyme. Results demonstrated that all honey samples (the darker and the lighter) and in all concentrations were highly effective against O−2. All honey samples exhibited more or less the same high antioxidant activity in X–XOD assay within the concentration 1 mg honey/1 ml. In contrast, the concentration (10 mg honey/ml) showed a lower activity than that of (1 mg honey/ml). Here, we demonstrated for the first time a significant inverse correlation between dilution and antioxidant activity, where the concentration 1 mg honey/ml >10 mg honey/ml >0.1 mg honey/ml (Fig. 2). Inoue et al. (30) demonstrated that only manuka honey had specific scavenging activity for superoxide anion radicals.
Superoxide anion radical (O−2) has been of intense interest owing to its increased dominance in vivo in different disease conditions (inflammation, cancer and atherosclerosis) (3). The compound possessing both (O−2) scavenging as well as XOD inhibitory activity may offer better therapeutic potential. Flavonoids with both these properties possess in common hydroxyl groups either at C-5, C-3 or C-3′ and C-4′ (3).
Transition metals are powerful initiators of lipid peroxidation. It was observed that several aldehydes are formed, mainly the 4-hydroxy-2-nonenal and malondialdehyde (MDA) (31). The formation of MDA was monitored by measuring the TBARS (it was done in vitro for the first time). In the LDL peroxidation assay, the results clarified that all honey samples (darker and lighter) and in all concentrations were highly effective against LDL peroxidation. The results are more or less near to control results. All the honey types under this experiment exhibited more or less the same high antioxidant activity within the concentration of 1 mg honey/1 ml (i.e. it has the same result of the control). Here, we demonstrated for the first time a significant inverse correlation between dilution and antioxidant activity, where the concentration 1 mg honey/ml > 10 mg honey/ml > 0.1 mg honey/ml > 100 mg honey/ml (Fig. 3).
Gheldof and Engeseth (10) analyzed in vitro the inhibition of lipoprotein oxidation by honeys from seven different floral sources. This was done by monitoring the conjugated diene formation directly on serum and not specifically on LDL. They studied the effect of the concentration 1 mg honey/1 ml for darker honeys and 2 mg honey/1 ml for lighter honeys. The darkest colored honeys, such as buckwheat honey, had the highest inhibitory activity. Although the results of Gheldof et al. (11) showed that the serum antioxidant capacity increased significantly by 7% following consumption of buckwheat honey in water, their ex vivo studies of serum lipoprotein oxidation and TBARS values were not significantly altered after consumption of any of the five beverages including buckwheat honey in water. This in vitro result could be attributed to the degree of dilution of honey (160 mg/ml) because in their previous in vitro study the darker honeys had the highest inhibition activity by using the concentration 1 mg honey/1 ml (10). So this in vivo study proved that honey has an effective antioxidant capacity.
They also found that the oxygen radical absorbance capacity (ORAC) values of honey (3–17 μmol TE/g) were in the same range as ORAC values of many fruits and vegetables (0.5–19 μmol TE/g fresh weight). These results indicate that honey is comparable to fruits and vegetables in antioxidant capacity on a fresh weight basis.
So our work is in agreement with Gheldof and Engeseth (10) and Gheldof et al. (11) works, who found that dilution to 1 mg honey/1 ml is highly effective, while 160 mg honey/1 ml had no antioxidant activity.
The highest activity of the concentration, 1 mg honey/1 ml, could be attributed to the increasing activity of glucose oxidase enzyme by dilution (honey pH 3.9–6.1, the optimum pH for glucose oxidase activity is 6.5–8) (32); increasing activity of glucose oxidase enzyme lead to the increase of the concentration of hydrogen peroxide. The more hydrogen peroxide is generated the more potent is the radical trapping (3).
Flavonoids are known to inhibit LDL oxidation through both metal chelation and free radical scavenging mechanisms, whereas phenolic acids act as antioxidant by free radical trapping mechanism (33). Flavonoids protecting (LDL) against Cu2+ ion-induced oxidation are dependent on their structural properties. The fewer the number of OH groups, the lower the probability of hydrogen loss and the lower the probability of oxidation of the flavonoid and the reduction of the metal. Whether chelation or oxidation, their partitioning abilities between the aqueous compartment and the lipophilic environment within the LDL particle, and their hydrogen-donating antioxidant properties are important aspects. It is clear from Tables 1 and 2 that Palm honey is the richest one in aromatic acids and Coriander and Sider honeys are the richest in flavonoids.
Many other components that have not been investigated in the present study might also contribute to total antioxidant activity. Salicylic acid, for example, has been found in honey (34) and is known to neutralize oxygen free radicals (35). Different amounts and types of minerals can also influence the antioxidant activity of the honeys. The mineral content varies in honeys from ∼0.04% in pale honeys to 0.2% in some dark honey samples (36).
In general, Gheldof et al. (10) reported that, the antioxidant capacity of honey appeared to be a result of the combined activity of a wide range of compounds including phenolics, peptides, organic acids, enzymes and possibly other minor components. The phenolic compounds contributed significantly to the antioxidant capacity of honey but were not solely responsible for it.
To illustrate the differences in honey phenolics due to the geographical origin (propolis-derived phenolics) and the similarities between floral derived phenolics of monofloral honey samples, clearly that the profiles are quite different. The GC/MS and HPLC analysis of Palm honey revealed similarities with palm propolis in hydroxyacetic acid, 3-hydroxypropanoic acid, malic acid, palmitic acid, 4-hydroxybenzoic acid, 3,4-dimethoxy-cinnamic acid, Caffeic acid, glycerol and phosphoric acid (37) and quercetin, quercitin-3-methylether, quercetin-3,3′-dimethylether and apigenin (38). Also Palm honey revealed similarities with date palm in 4-OH benzoic, vanillic, caffeic, p-coumaric acids and quercitin, quercitin-3-methyl ether (39,40). Acacia honey revealed similarities with acacia wood only in vanillic acid (41). The hydroxycinnamates, caffeic, p-coumaric and ferulic acids were found in European Acacia honeys (18). In our investigation cinnamic and cis-p-coumaric acids were only identified in Acacia honey.
Our study provides (for the first time) primary evidence suggesting that these honeys in further in vivo studies could play an important role in inhibiting lipid peroxidation in biological systems through their antioxidant, metal chelating and free radical scavenging activities. Also some bee products as propolis contain a higher level of phenolic compounds and showed strong capability to scavenge free radicals and exhibit a cytotoxic effect on human melanoma cells (42). It also induced inhibition of oxidative stress which may be partly responsible for its neuroprotective function against in vitro cell death and in vivo focal cerebral ischemia (43). So the use of bee products have mainly centered on prevention and the maintenance of human health.
Acknowledgement
The authors are grateful for the financial support by the National Research Center of Egypt (Contract 3/23/6 and 1/48/5). Also grateful for Prof. Dr E. Wollenweber (Darmstadt, Germany) for providing many authentic samples of flavonoids and Dr Kamel H. Shaker, National Research Center for providing RP-18 column. Also grateful for El-Yahia Company, Saudi Arabia for providing Sider honey.
References
- 1.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem. 1998;46:4113–7. [Google Scholar]
- 2.Fejes S, Kery A, Blazovics A. Investigation of the in-vitro antioxidant activity of Petroselinum crispum (Mill.) Nym.exA.W.Hill. Acta Pharm Hung. 1998;68:150–6. [PubMed] [Google Scholar]
- 3.Tiwari AK. Imbalance in antioxidant defense and human diseases: Multiple approach of natural antioxidants therapy. Curr Sci India. 2001;81:1179–87. [Google Scholar]
- 4.Fuhrman B, Rosenblant M, Hayek T. Ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. J Nutr. 2000;130:1124–31. doi: 10.1093/jn/130.5.1124. [DOI] [PubMed] [Google Scholar]
- 5.Aviram M, Dornfeld L, Kaplan M. Pomegranate juice flavonoids inhibit LDL oxidation and cardiovascular diseases: Studies in atherothecrotic mice and humans. Drugs Exp Clin Res. 2002;28:49–62. [PubMed] [Google Scholar]
- 6.Stocker R, Keaney JF., Jr Role of oxidative modification in atherosclerosis. Physiol Rev. 2004;84:1381–478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 7.Visioli F, Bellomo G, Montedoro GF, Galli G. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis. 1995;117:25–32. doi: 10.1016/0021-9150(95)05546-9. [DOI] [PubMed] [Google Scholar]
- 8.Kamiya K, Tanaka Y, Endang H. Chemical constituents of Morinda citrifolia fruits inhibit cupper-induced low-density lipoprotein oxidation. J Agric Food Chem. 2004;52:5843–8. doi: 10.1021/jf040114k. [DOI] [PubMed] [Google Scholar]
- 9.Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 2002;50:5870–7. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- 10.Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 2002;50:5870–7. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- 11.Gheldof N, Wang XH, Engeseth NJ. Buckwheat honey increases serum antioxidant capacity in humans. J Agric Food Chem. 2003;51:1500–5. doi: 10.1021/jf025897t. [DOI] [PubMed] [Google Scholar]
- 12.Schramm DD, Karim M, Schrader HR, Holt RR, Cardetti M, Keen CL. Honey with high levels of antioxidants can provide protection to healthy human subjects. J Agric Food Chem. 2003;51:1732–5. doi: 10.1021/jf025928k. [DOI] [PubMed] [Google Scholar]
- 13.Frankel S, Robinson GE, Berenbaum MRL. Antioxidant content and correlated characteristics of 14 monofloral honeys. J Apic Res. 1998;37:27–31. [Google Scholar]
- 14.Ferreres F, Ortiz A, Silva C, García-Viguera C, Tomás- Barberan FA, Tomás-Lorente F. Flavonoids of ‘La Alcarria’ honey. Z Lebensm Unters Forsch. 1992;194:139–43. [Google Scholar]
- 15.Andrade P, Ferreres F, Amaral MT. Analysis of honey phenolic acids by HPLC, its application to honey botanical characterization. J Liq Chromatogr Relat Technol. 1997;20:2281–8. [Google Scholar]
- 16.Ferreres F, Tomás-Barberán FA, Soler C, García-Viguera C, Ortiz A, Tomás Lorente F. A simple extractive technique for honey flavonoid HPLC analysis. Apidologie. 1994;25:21–30. [Google Scholar]
- 17.Martos I, Ferreres F, Tomás-Barberán FA. Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J Agric Food Chem. 2000;48:1498–502. doi: 10.1021/jf991166q. [DOI] [PubMed] [Google Scholar]
- 18.Tomás-Barberán FA, Martos I, Ferreres F, Radovic BS, Anklam E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J Sci Food Agric. 2001;81:485–96. [Google Scholar]
- 19.Louveaux J, Maurizio A, Vorwohl G. Methods of melissopalynology. Bee World. 1978;59:139–57. [Google Scholar]
- 20.Tan S-T, Wilkins AL, Holland PT, McGhie TK. Extractives from New Zealand honeys. 3. Unifloral Thyme and Willow honey constituents. J Agric Food Chem. 1990;38:1833–8. [Google Scholar]
- 21.Greenaway W, May J, Scaysbrook T, Whatley FRS. Identification by gas chromatography mass spectrometry of 150 compounds in propolis. Z Naturforsch. 1991;46c:111–21. [Google Scholar]
- 22.Matsushige K, Basnet P, Kadota S, Namba T. Potent free radical scavenging activity of dicaffeoyl quinic acid derivatives from propolis. J Traditional Med. 1996;13:217–28. [Google Scholar]
- 23.Gugliucci A, Menini T. Three different pathways for human LDL oxidation are inhibited in vitro by water extracts of the medicinal herb Achyrocline satureoides. Life Sci. 2002;71:693–705. doi: 10.1016/s0024-3205(02)01734-4. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 25.Masaki N, Kyle ME, Faber JL. tert-Butyl Hydroperoxides kills cultured hepatocytes by peroxidizing membrane lipids. Arch Biochem Biophys. 1989;269:390–9. doi: 10.1016/0003-9861(89)90122-7. [DOI] [PubMed] [Google Scholar]
- 26.Ogan K, Katz E. Liquid chromatographic separation of alkyl phenols with fluorescence and ultra violet detection. Anal Chem. 1981;53:160–3. [Google Scholar]
- 27.Annual book of ASTM Standards, part 11.03 Atmospheric analysis. American Scosity for testing and materials, Philadelphia, Pennsylvania. 1983.
- 28.Senedcor GW. Statistical Methods. 5th. Iowa USA: Iowa State University Press; 1961. [Google Scholar]
- 29.Fuhrman B, Aviram M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr Opin Lipidol. 2001;12:41–8. doi: 10.1097/00041433-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Koichi I, Shiho M, Fumie S, Kazue T, Yoshihiro Y, Hiroyuki N. Identification of phenolic compound in manuka honey as specific superoxide anion radical scavenger using electron spin resonance (ESR) and liquid chromatography with coulometric array detection. J Sci Food Agric. 2005;85:872–8. [Google Scholar]
- 31.Uchida K, Toyokuni S, Nishikawa K. Michael addition type 4-hydroxy- 2-nonenal adducts in modified low-density lipoproteins: Markers for atherosclerosis. Biochemistry. 1994;33:12487–94. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- 32.Molan PC. The antibacterial activity of honey. 1. The nature of the antibacterial activity. Bee World. 1992;73:5–28. [Google Scholar]
- 33.Hall CA, III, Cuppett SL. Structure-activities of natural antioxidants. In: Aruoma OI, Cuppett SL, editors. Antioxidant Methodology: In Vivo and In Vitro Concepts. Champaign, IL: AOCS Press; 1997. pp. 141–72. [Google Scholar]
- 34.Venema DP, Hollman PCH, Janssen KPLTM, Katan MB. Determination of acetylsalicylic acid and salicylic acid in foods, using HPLC with fluorescence detection. J Agric Food Chem. 1996;44:1762–7. [Google Scholar]
- 35.Scheier L. Salicylic acid: One more reason to eat your fruits and vegetables. J Am Diet Assoc. 2001;101:1406–8. doi: 10.1016/S0002-8223(01)00337-6. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Otero JL, Paseiro P, Simal J, Cepeda A. Mineral content of the honeys produced in Galicia (North-west Spain) Food Chem. 1994;49:169–71. [Google Scholar]
- 37.Hegazi AG, Abd El Hady FK. Egyptian propolis: 3- Antioxidant, antimicrobial activity and chemical composition of propolis from reclaimed land. Z Naturforsch. 2002;57c:395–402. doi: 10.1515/znc-2002-3-432. [DOI] [PubMed] [Google Scholar]
- 38.Hegazi AG, Faten K, Abd El-Hady. Egyptian propolis: 8- Antiviral and antioxidant activities in relation to flavonoid content. 1st International Forum on Apitherapy. 2006:25–26. Athens 12–15 October. [Google Scholar]
- 39.Regnault-Roger C, Hadidan R, Biard JF, Boukef K. High performance liquid and thin-layer chromatographic determination of phenolic acids in palm (Phoenix dactilifera) products. Food Chem. 1987;25:61–71. [Google Scholar]
- 40.Ziouti A, El-Bboustani E, Macheix JJ, Geibel M, Treutter D, Feuch W. Cell wall- bound phenols in date palm leaves and roots. Acta- Horticulturae. 1994;381:276–9. [Google Scholar]
- 41.Miyazawa M, Shimamura H, Nakamura S, Kameoka H. Partial suppression of SOS-inducing activity of furylfuramide by dibasic acids from Ipomoea nil in the Salmonella typhimurium TA1535/Psk1002 umu test. J Agric Food Chem. 1995;43:284–7. [Google Scholar]
- 42.Chen CN, Weng MS, Wu CL, Lin JK. Comparison of radical scavenging activity, cytotoxic effects and apoptosis induction in human melanoma cells by Taiwanese propolis from different sources. eCAM. 2004;1:175–85. doi: 10.1093/ecam/neh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimazawa M, Chikamatsu S, Morimoto N, Mishima S, Nagai H, Hara H. Neuroprotection by Brazilian green propolis against in vitro and in vivo ischemic neuronal damage. eCAM. 2005;2:201–7. doi: 10.1093/ecam/neh078. [DOI] [PMC free article] [PubMed] [Google Scholar]