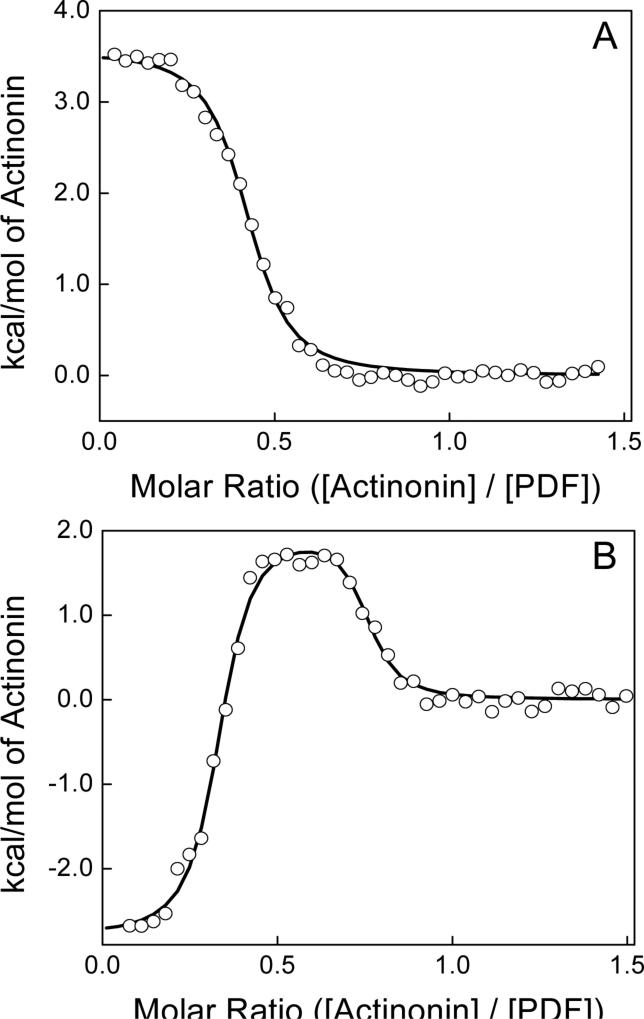
Figure 3.
Comparison of the binding isotherms for Ni2+-free (panel A) and ZnCl2-treated PDFEc (panel B) titrated with 325 μM actinonin in 50 mM potassium phosphate, pH 7.0 at 25 °C. Total protein concentrations were 31.5 μM in both experiments and the best fit of single binding site model (solid line in panel A) provided n, Ka and ΔH° of 0.41, 2.7 × 106 and 3.6 kcal mol−1, respectively for Ni2+-free PDFEc. The solid line in panel B corresponds to the best fit of the ZnCl2-treated PDFEc experimental data to an independent two-site model providing n of 0.31 and 0.42, Ka of 3.9 × 108 and 5.7 × 106 M−1 and ΔH° of −2.8 and 2.0 kcal mol−1 for the high and low affinity binding sites, respectively.