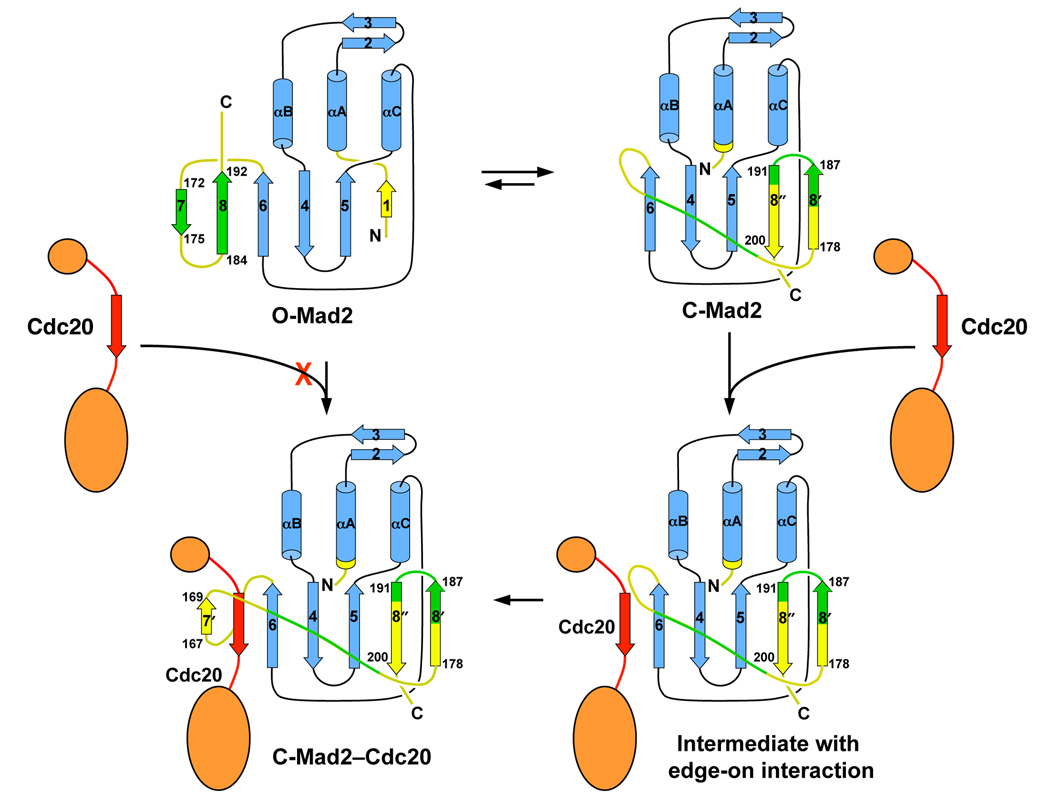
Figure 4. A Schematic Model Explaining Why O-Mad2 Is an Autoinhibited Conformer.
In O-Mad2, the C-terminal β7/8 hairpin blocks the access of Cdc20 to β6. In C-Mad2, β6 is exposed and can allow edge-on interactions with he Mad2-binding motif of Cdc20. The β8’/8’’ hairpin can then detach from β5, wrap around Cdc20, and re-attach to β5. In the Mad2–Cdc20 complex, Cdc20 is trapped by Mad2, similar to the way that car passengers are restrained by seat-belts. Note that both the original β7/8 hairpin and the corresponding regions in C-Mad2 are colored green. The hydrogen-bonding networks in the β7/8 and β8’/8’’ hairpins are different from one another.