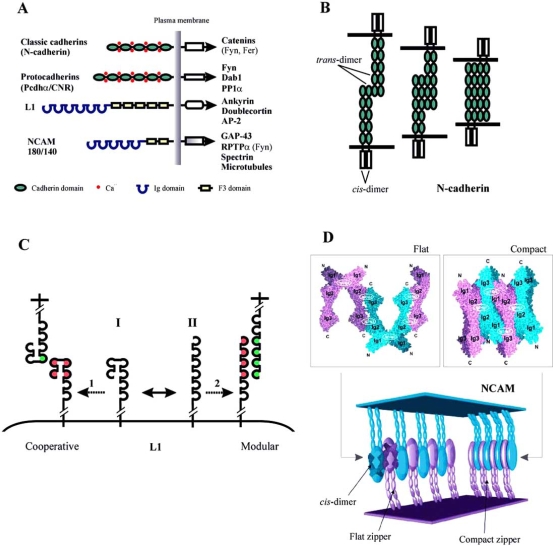
Fig. (1).
Structural basis of CAM-mediated cell adhesion. (A)Domain structure of classic and proto- cadherins, L1, and NCAM 180/140. Binding partners for cytoplasmic tails of CAMs are shown. (B) A model of cadherin-mediated cell adhesion (shown for N-cadherin). (C) Two proposed mechanisms of homophilic L1 binding. Note that the cartoon of L1 is shortened to highlight Ig1-Ig6. The horseshoe (I) and the extended (II) conformations of L1 exist in dynamic equilibrium and may underlie two different mechanisms of homophilic L1 binding, occurring in the cooperative (1) or modular (2) mode. See text for details. (D)A model of homophilic NCAM binding. Top: crystal structure of NCAM Ig1-Ig2-Ig3 showing interaction between the three Ig-modules. Bottom: organization of the extracellular part of NCAM molecules engaged in both a flat and a compact zipper. The large ellipsoids correspond to the two interacting Ig1-Ig2-Ig3 constructs as shown in the leftmost part (modified from [73]).