Figure 3.
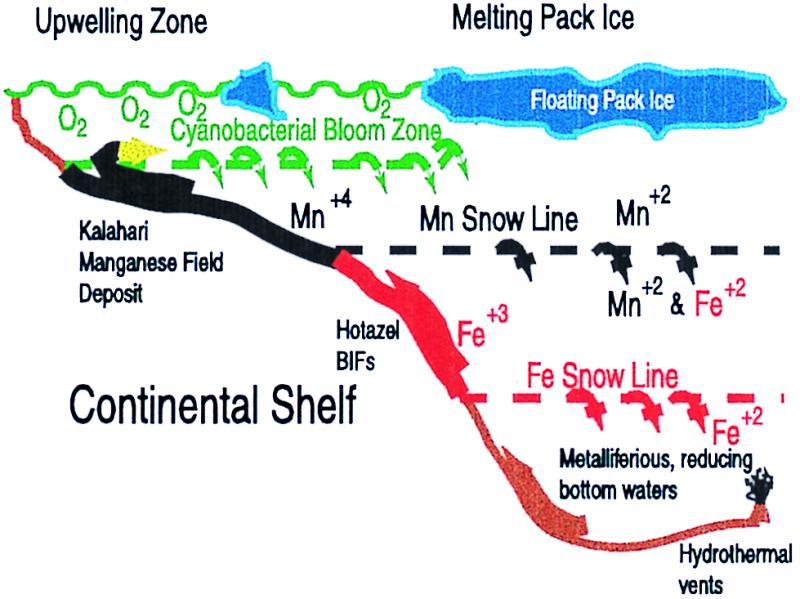
Schematic setting for deposition of the Kalahari Manganese Field in a postsnowball environment. Upon initial melting, the cyanobacterial bloom exposes the anoxic, metalliferous waters to free O2 in the euphotic zone. Initially, both Mn+4 and Fe+3 precipitate out and settle gravitationally. However, when the Mn+4 sinks to waters still containing Fe+2 in solution, the manganese will be re-reduced to the soluble Mn+2, generating more Fe+3 precipitate. (This reaction may be biologically mediated.) Eventually, the iron is stripped out of the surface zone, forming the Mn “snow line” as indicated. Continued upwelling of waters on the edge of the platform should increase the Mn concentration, allowing Mn precipitates to collect on the platform, producing the Kalahari manganese ore deposits in the Hotazel Formation. Below the Mn snow line, both Fe+2 and Mn+2 would be in solution whereas Fe+3 minerals are precipitating to form the iron stones. If the bottom waters are reducing enough, there may even be an Fe snow line, below which the Fe+3 is reduced and returned to solution. (No sediments from this depth appear to be preserved in the Kalahari.) Small changes in relative sea level could then cause these metal snow lines to move up and down the platform, yielding some of the lateral and temporal variations in the Kalahari manganese field deposits (23).
