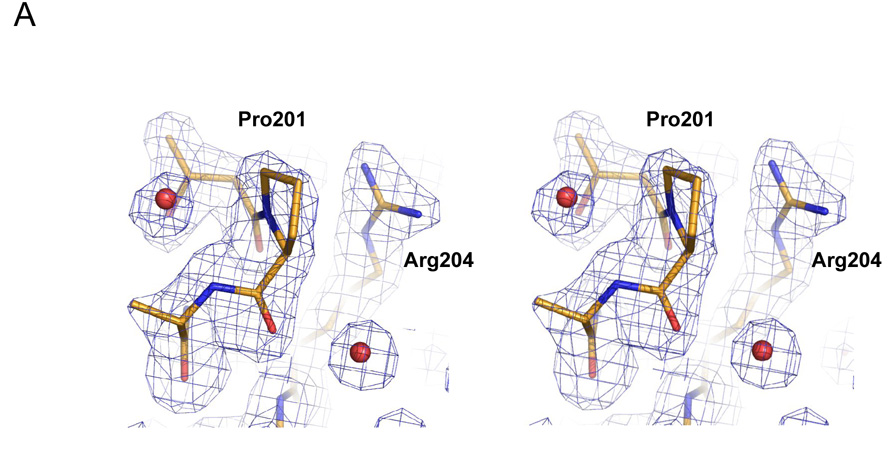
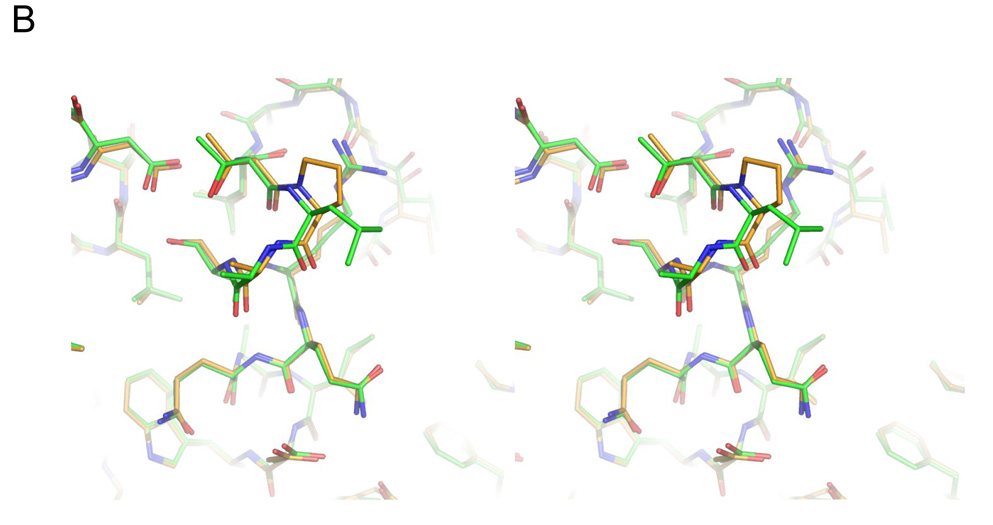
Figure 4.
Structure of the L201P mutant of TEM-1 β-lactamase. A. 2Fo-Fc electron density map, contoured at 1σ, shows the mutated residue 201 and surrounding environment, including two ordered water molecules (red spheres) that interact with the protein backbone. Carbon atoms are colored in gold, oxygen atoms in red and nitrogen atoms in blue. B. Overlay of the TEM-1 WT structure (carbons in green) and the L201P mutant (carbons in gold) in the region of the Leu201 → Pro substitution. C. Wild-type TEM-1 (1AXB) helix 9 is shown next to α9 of the L201P structure. The residue at position 201 is presented in stick format and the solvent exposed surface associated with residue 201 is presented in each structure.