Abstract
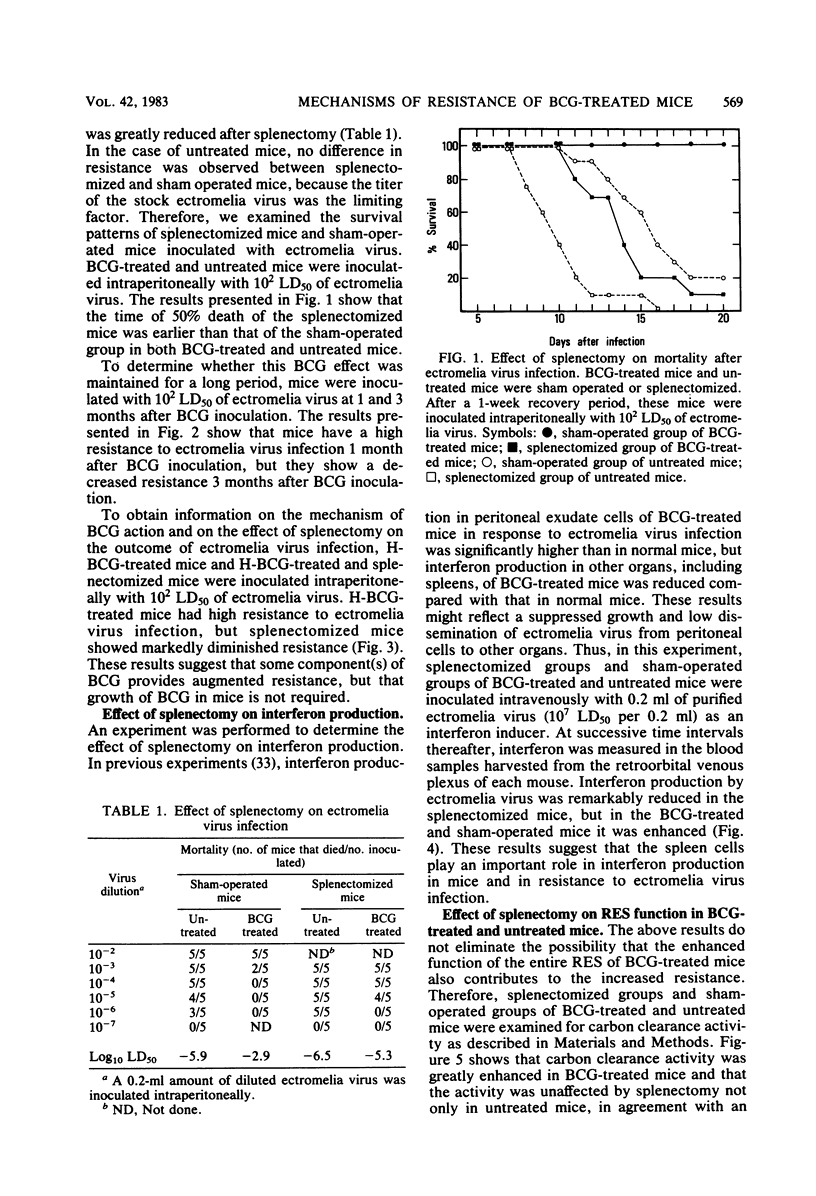
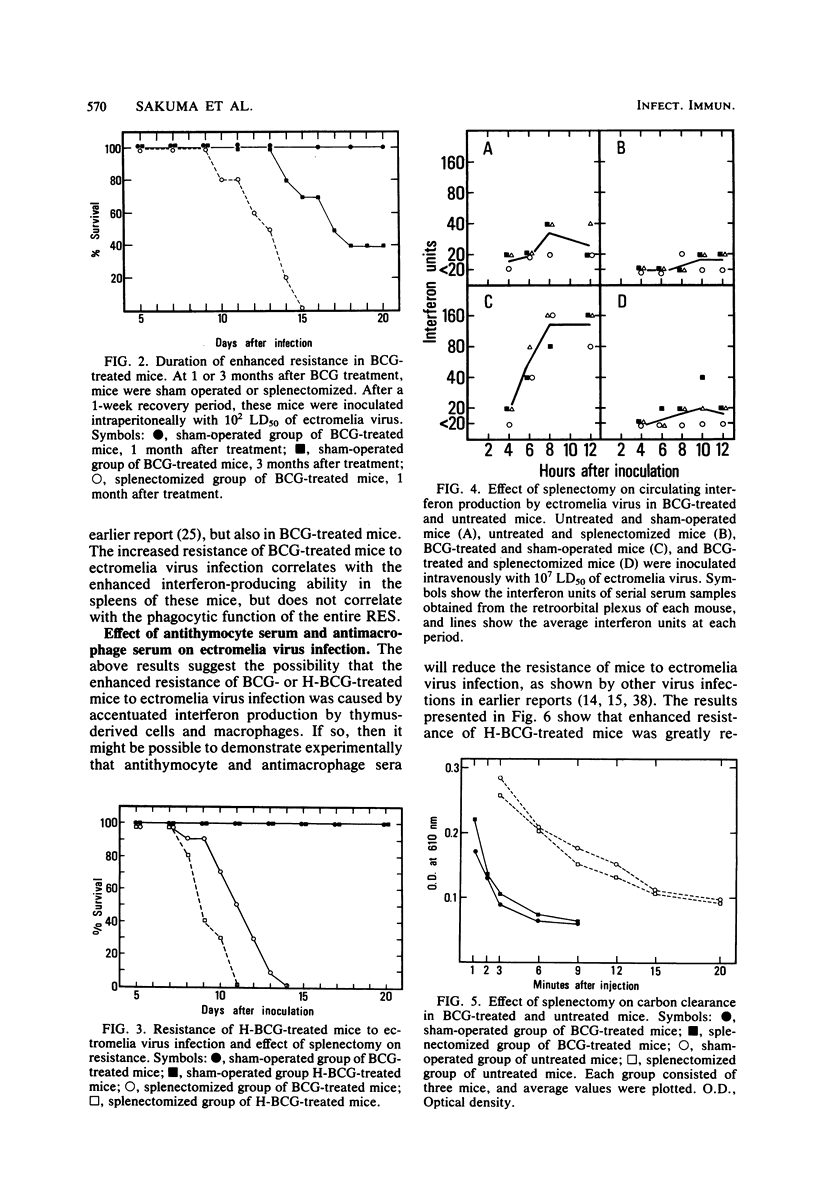
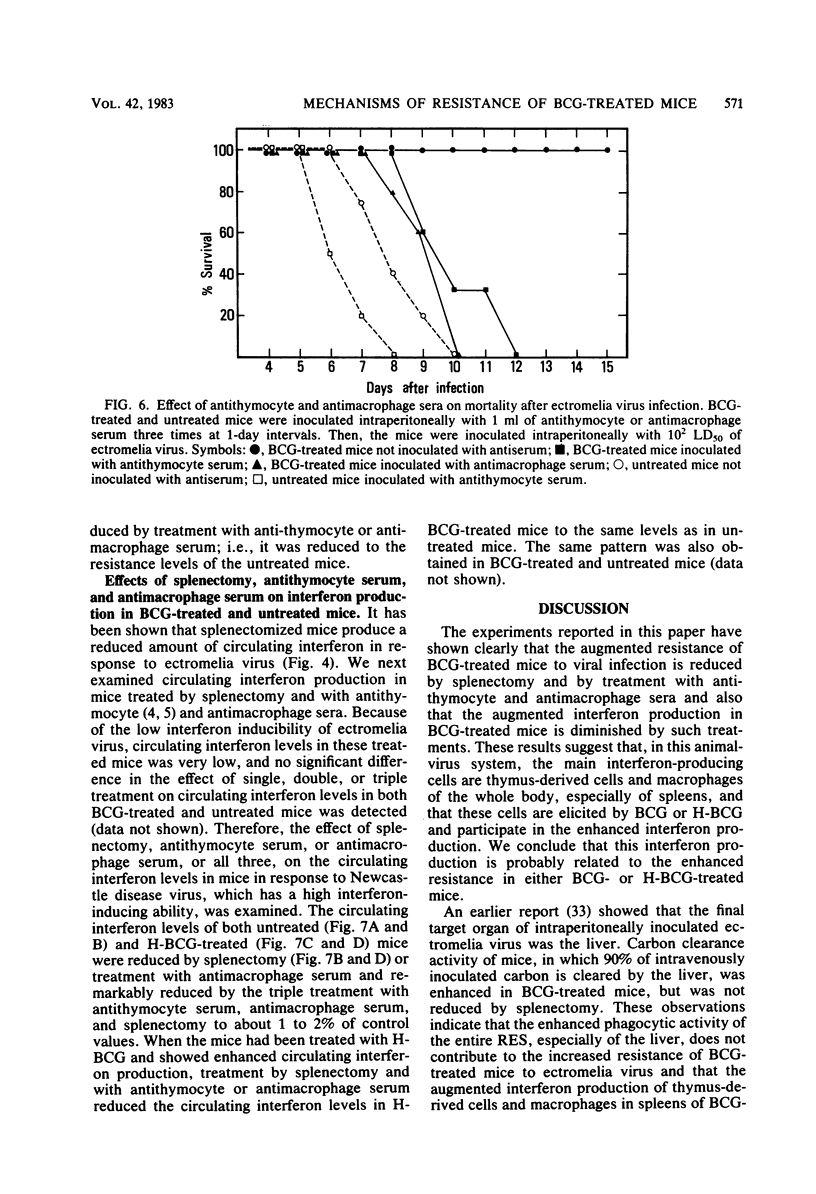
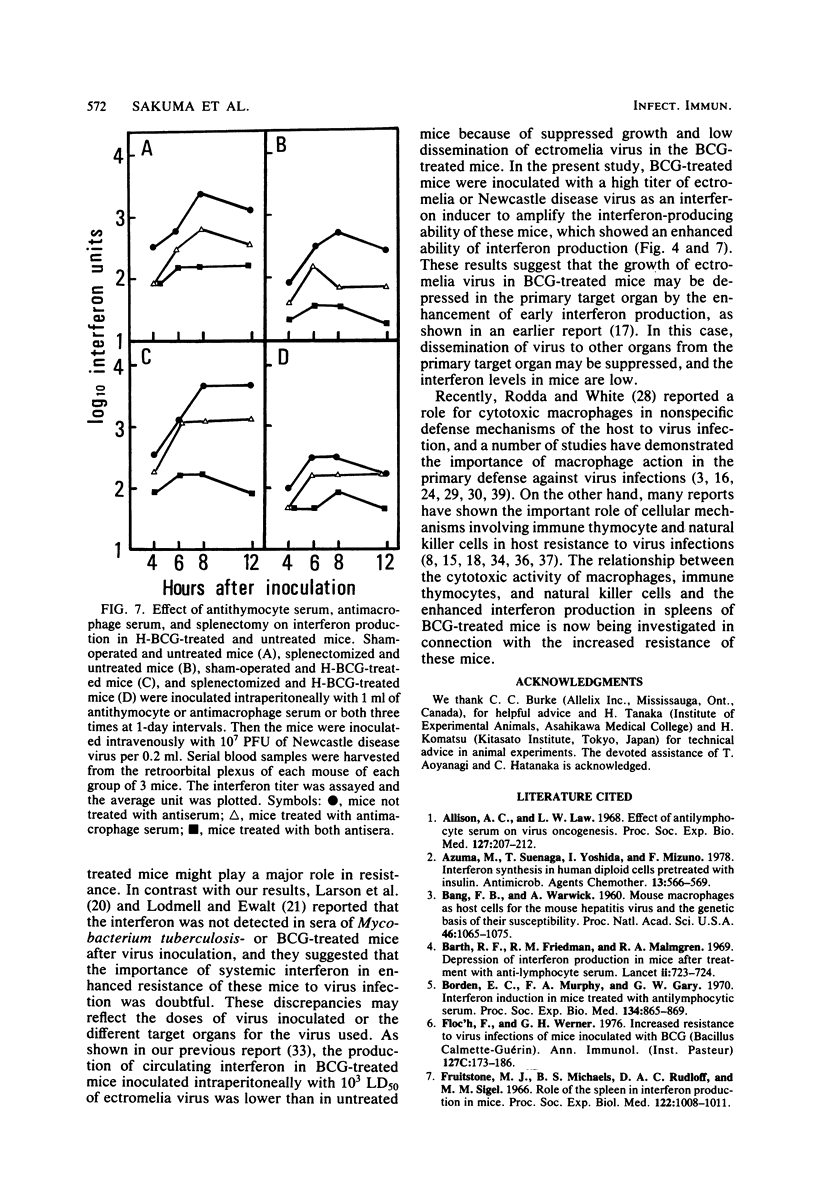
The mechanism of enhanced resistance of Mycobacterium bovis BCG-treated mice to ectromelia virus infection was investigated by determining the effect of splenectomy, antithymocyte serum, and antimacrophage serum on resistance. It was greatly reduced by these treatments, not only in normal mice, but also in mice treated with live or heat-inactivated BCG. Production of circulating interferon by ectromelia virus and Newcastle disease virus was augmented in BCG-treated mice and was markedly depressed by splenectomy and antithymocyte and antimacrophage serum treatments in both BCG-treated and normal mice. Carbon clearance activity was activated in BCG-treated mice, but splenectomy did not influence phagocytic activity. These results suggest that augmented interferon production in the spleens of BCG-treated mice plays a major role in enhanced resistance. Other possible mechanisms are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Law L. W. Effects of antilymphocyte serum on virus oncogenesis. Proc Soc Exp Biol Med. 1968 Jan;127(1):207–212. doi: 10.3181/00379727-127-32657. [DOI] [PubMed] [Google Scholar]
- Azuma M., Suenaga T., Yoshida I., Mizuno F. Interferon synthesis in human diploid cells pretreated with insulin. Antimicrob Agents Chemother. 1978 Apr;13(4):566–569. doi: 10.1128/aac.13.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth R. F., Malmgren R. A., Friedman R. M. Depression of interferon production in mice after treatment with anti-lymphocyte serum. Lancet. 1969 Oct 4;2(7623):723–724. doi: 10.1016/s0140-6736(69)90431-0. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Murphy F. A., Gary G. W., Jr Attempted suppression of the interferon response in the mouse. Proc Soc Exp Biol Med. 1970 Jul;134(3):865–869. doi: 10.3181/00379727-134-34900. [DOI] [PubMed] [Google Scholar]
- Floc'h F., Werner G. H. Increased resistance to virus infections of mice inoculated with BCG (Bacillus calmette-guérin). Ann Immunol (Paris) 1976 Mar-Apr;127(2):173–186. [PubMed] [Google Scholar]
- Fruitstone M. J., Michaels B. S., Rudloff D. A., Sigel M. M. Role of the spleen in interferon production in mice. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1008–1011. doi: 10.3181/00379727-122-31311. [DOI] [PubMed] [Google Scholar]
- GLEDHILL A. W., REES R. J. Effect of a primary tuberculous infection on the resistance of male and female mice to Ectromelia. Nature. 1960 Aug 20;187:703–704. doi: 10.1038/187703b0. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A. Cellular immunity in host resistance to viral infections. Arch Intern Med. 1970 Jul;126(1):125–134. [PubMed] [Google Scholar]
- Gorhe D. S. Inhibition of multiplication of foot and mouth disease virus in adult mice pretreated with Freund's complete adjuvant. Nature. 1967 Dec 23;216(5121):1242–1244. doi: 10.1038/2161242a0. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bandu M. E., Maury C., Brouty-Boyé D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976 Nov 2;144(5):1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Maury C., Bandu M. T. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. II. Studies with herpes simplex, Moloney sarcoma, vesicular stomatitis, Newcastle disease, and influenza viruses. J Exp Med. 1976 Nov 2;144(5):1316–1323. doi: 10.1084/jem.144.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser J., Morel-Maroger L., Verroust P., Rivière Y., Guillon J. C. Anti-interferon globulin inhibits the development of glomerulonephritis in mice infected at birth with lymphocytic choriomeningitis virus. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3413–3416. doi: 10.1073/pnas.75.7.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Murphy F. A. Effects of anti-lymphoid sera on viral infections. Lancet. 1968 Jul 6;2(7558):37–40. doi: 10.1016/s0140-6736(68)92904-8. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Nahmias A. J., Murphy F. A., Kramer J. H. Cellular immunity in vaccinia infection of mice. Anti-thymocyte serum effects on primary and secondary responsiveness. J Exp Med. 1968 Jul 1;128(1):121–132. doi: 10.1084/jem.128.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Iwasaki T., Nozima T. Defense mechanisms against primary influenza virus infection in mice. I. The roles of interferon and neutralizing antibodies and thymus dependence of interferon and antibody production. J Immunol. 1977 Jan;118(1):256–263. [PubMed] [Google Scholar]
- Kirchner H., Engler H., Zawatzky R., Schindler L. Role of natural killer cells and of interferon in natural resistance against virus infections. Adv Exp Med Biol. 1982;155:785–797. doi: 10.1007/978-1-4684-4394-3_86. [DOI] [PubMed] [Google Scholar]
- Larson C. L., Ushijima R. N., Karim R., Baker M. B., Baker R. E. Herpesvirus hominis type 2 infections in rabbits: effect of prior immunization with attenuated Mycobacterium bovis (BCG) cells. Infect Immun. 1972 Oct;6(4):465–468. doi: 10.1128/iai.6.4.465-468.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Ewalt L. C. Enhanced resistance against encephalomyocarditis virus infection in mice, induced by a nonviable Mycobacterium tuberculosis oil-droplet vaccine. Infect Immun. 1978 Jan;19(1):225–230. doi: 10.1128/iai.19.1.225-230.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Ewalt L. C. Induction of enhanced resistance against encephalomyocarditis virus infection of mice by nonviable Mycobacterium tuberculosis: mechanisms of protection. Infect Immun. 1978 Dec;22(3):740–745. doi: 10.1128/iai.22.3.740-745.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara N., Nagano Y. Formation of virus-inhibiting factor or interferon in splenectomized mice. Jpn J Microbiol. 1972 Nov;16(6):469–474. doi: 10.1111/j.1348-0421.1972.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Mogensen S. Role of macrophages in hepatitis induced by Herpes simplex virus types 1 and 2 in mice. Infect Immun. 1977 Mar;15(3):686–691. doi: 10.1128/iai.15.3.686-691.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano Y., Komatsu H. Effect of activation of the reticuloendothelial system on formation of virus-inhibiting factor or interferon. Jpn J Microbiol. 1975 Jun;19(3):228–231. doi: 10.1111/j.1348-0421.1975.tb00873.x. [DOI] [PubMed] [Google Scholar]
- Pusateri A. M., Ewalt L. C., Lodmell D. L. Nonspecific inhibition of encephalomyocarditis virus replication by a type II interferon released from unstimulated cells of Mycobacterium tuberculosis-sensitized mice. J Immunol. 1980 Mar;124(3):1277–1283. [PubMed] [Google Scholar]
- Rivière Y., Gresser I., Guillon J. C., Tovey M. G. Inhibition by anti-interferon serum of lymphocytic choriomeningitis virus disease in suckling mice. Proc Natl Acad Sci U S A. 1977 May;74(5):2135–2139. doi: 10.1073/pnas.74.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda S. J., White D. O. Cytotoxic macrophages: a rapid nonspecific response to viral infection. J Immunol. 1976 Dec;117(6):2067–2072. [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Defense mechanisms against infectious bovine rhinotracheitis virus: inhibition of virus infection by murine macrophages. Infect Immun. 1975 Mar;11(3):505–511. doi: 10.1128/iai.11.3.505-511.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyan T. P., Mims C. A. Fate of intravenously administered interferon and the distribution of interferon during virus infections in mice. Br J Exp Pathol. 1966 Apr;47(2):168–176. [PMC free article] [PubMed] [Google Scholar]
- Suenaga T., Okuyama T., Yoshida I., Azuma M. Effect of Mycobacterium tuberculosis BCG infection on the resistance of mice to ectromelia virus infection: participation of interferon in enhanced resistance. Infect Immun. 1978 Apr;20(1):312–314. doi: 10.1128/iai.20.1.312-314.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake H., Inada T. Mechanism of induction of cellular immunity to virus infections: experiments with adenoviruses. Ann Microbiol (Paris) 1977 Nov-Dec;128B(4):517–530. [PubMed] [Google Scholar]
- Virelizier J. L., Gresser I. Role of interferon in the pathogenesis of viral diseases of mice as demonstrated by the use of anti-interferon serum. V. Protective role in mouse hepatitis virus type 3 infection of susceptible and resistant strains of mice. J Immunol. 1978 May;120(5):1616–1619. [PubMed] [Google Scholar]
- Welsh R. M. Natural cell-mediated immunity during viral infections. Curr Top Microbiol Immunol. 1981;92:83–106. doi: 10.1007/978-3-642-68069-4_6. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F., Toy S. T. Participation of lymphocytes in viral infections. Adv Immunol. 1973;16:123–184. doi: 10.1016/s0065-2776(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- Zisman B., Wheelock E. F., Allison A. C. Role of macrophages and antibody in resistance of mice against yellow fever virus. J Immunol. 1971 Jul;107(1):236–243. [PubMed] [Google Scholar]


