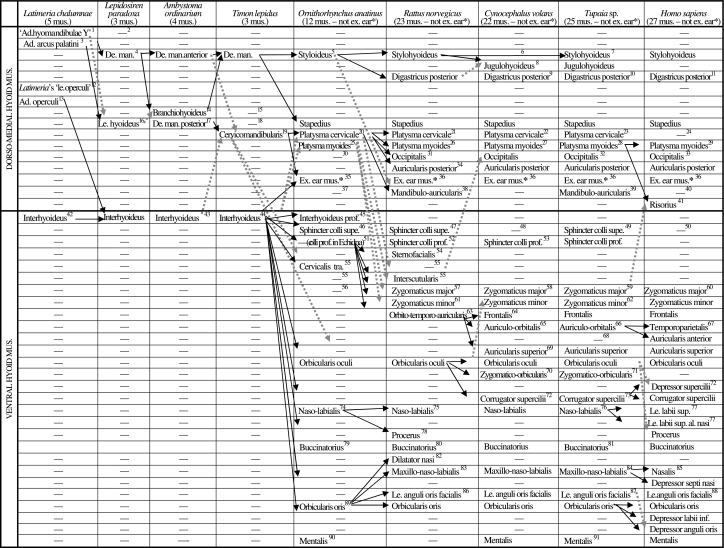
Table 2.
Scheme illustrating the authors’ hypotheses regarding the homologies of the hyoid muscles of adults of representative sarcopterygian taxa (see caption of Table 2, text, and Figs 3–17; ad. = adductor; al. = alaeque; de. man. = depressor mandibulae; ex. = extrinsic; inf. = inferioris; le. = levator; mus. = muscles; prof. = profundus; sup. = superioris; supe. = superficialis; tra. = transversus)
1[seemingly not homologous with the adductor hyomandibulae of actinopterygians such as teleosts: Diogo 2007, 2008]
2[seemingly absent in dipnoans and tetrapods, although it may possibly be included in the levator hyoideus/depressor mandibulae: Diogo 2007, 2008]
3[the portion of the hyoid muscle anlage that gives rise to the levator hyoideus/depressor mandibulae of non-actinistian sarcopterygians probably corresponds to that giving rise to the adductor arcus palatini in other osteichthyans: Diogo 2007, 2008]
4[according to Forey 1986 the depressor mandibulae and levator hyoideus of extant dipnoans develop from the same ontogenetic anlage]
5 (the styloideus sensuHuber 1930a corresponds to the interhyoideus sensuEdgeworth 1935 and to the posterior digastric sensuParsons 1898) [Edgeworth 1935 suggested that the styloideus of monotremes and the stylohyoideus of other mammals derive from the interhyoideus; our observations and comparisons strongly support the interpretations of e.g. Huber 1930a and Saban 1968, 1971, i.e. that the monotreme styloideus and stapedius and the therian stylohyoideus, digastricus posterior, jugulohyoideus, stapedius and possibly mandibulo-auricularis correspond to the depressor mandibulae of reptiles such as Timon; Gasser's 1967 developmental study indicates that in modern humans the digastricus posterior, stapedius and stylohyoideus derive from a same anlage]
6[Saban 1968 states that the stylohyoideus is present in Cynocephalus but Gunnell & Simmons 2005 consider that this muscle is missing in this taxon; our dissections clearly indicate that the stylohyoideus is not present as an independent structure in adult colugos]
7[Sprague 1944a states that the styloglossus of certain tree-shrews is innervated by the hypoglossal nerve, but Le Gros Clark 1926 and Lightoller 1934 claim that this muscle is innervated by the facial nerve, as in other mammals]
8 (mastoideostyloideus sensuSaban 1968) [seemingly corresponds to part of the stylohyoideus and/or possibly of the digastricus posterior of mammals such as rats; see e.g. Huber 1930a, 1931; Saban 1968]
9 (part of biventer sensuLeche 1886) [our dissections indicate that the digastricus posterior and digastricus anterior of colugos are joined by a tendinous intersection, as described by e.g. Saban 1968]
10[our dissections indicate that the digastricus posterior and digastricus anterior of Tupaia are joined by a well-developed tendon, as described by e.g. Sprague 1944a]
11[the digastricus posterior and digastricus anterior of modern humans are usually joined by a well-developed tendon]
12[seemingly not homologous with the levator operculi of halecomorph and teleostean actinopterygians: Diogo, 2007, 2008]
13[its fibers are seemingly deeply mixed with those of the adductor arcus palatini: Diogo, 2007, 2008]
14[according to Edgeworth 1935 and Ericsson & Olsson 2004 the branchiohyoideus, interhyoideus and levator hyoideus appear at about the same time in urodele embryos, thus being difficult to infer if the branchiohyoideus is ontogenetically derived from the ventral or from the dorso-medial hyoid musculature; however, the developmental study of Piatt 1938 indicates that this muscle is in fact part of the dorso-medial hyoid musculature; it should be noted that Ambystoma ordinarium, as many other urodeles, has a muscle ceratomandibularis, which seems to derive from the same anlage that gives rise to the branchiohyoideus, but that, contrary to this latter muscle, is not present as an independent structure in the adult Ambystoma ordinarium specimens dissected by us; this muscle ceratomandibularis seems to correspond to the caecilian muscle ’hyomandibularis’ sensu Kleinteich & Haas 2007]
15[ the ’branchiohyoideus’ of reptiles is a branchial muscle that seemingly corresponds to the subarcualis rectus 1, and not to the hyoid muscle branchiohyoideus, of amphibians: e.g. Edgeworth 1935; Diogo 2007, 2008]
16[the portion corresponding to the levator hyoideus of dipnoans becomes attached on the mandible, forming the depressor mandibulae posterior; the depressor mandibulae anterior thus seemingly corresponds to the depressor mandibulae of dipnoans: Diogo 2007, 2008]
17[the Timon specimens dissected do not have an independent depressor mandibulae posterior nor an independent levator hyoideus but according to e.g. Edgeworth 1935 some adult reptiles do have a levator hyoideus]
18 (levator hyoideus sensuEdgeworth 1935) [the mammalian stapedius clearly derives from the levator hyoideus/depressor mandibulae of other tetrapods: e.g. Huber 1930a,b, 1931; Edgeworth 1935; Brocks 1938; Saban 1968, 1971; Kardong 2002]
19[our dissections and comparisons support the view of e.g. Huber 1930a and Edgeworth 1935, i.e. that this muscle corresponds to part of the depressor mandibulae/levator hyoideus of amphibians such as urodeles; see also the recent work of Tsuihiji 2007]
20 (the platysma cervicale sensuJouffroy and Saban 1971 corresponds to the pars nuchalis of the platysma sensuSaban 1971 and to part of the platysma sensuLightoller 1942) [our dissections and comparisons support the suggestion of authors such as Lightoller 1942 and Saban 1971, i.e. it seems likely that the reptilian sphincter colli (= interhyoideus) corresponds to the mammalian sphincter colli superficialis + sphincter colli profundus + their derivatives, and that the reptilian cervicomandibularis, which is more oblique than, and deep to, the interhyoideus, corresponds to the mammalian platysma + its derivatives; although the hypothesis that the facial musculature of mammals corresponds exclusively to the ventral (superficial) hyoid musculature of other tetrapods cannot be completely ruled out, it seems likely that at least some facial muscles may in fact be part of the dorso-medial, and not only of the ventral, hyoid musculature]
21[the cranial panniculus of Greene 1935 corresponds to our platysma cervicale + auriculolabialis inferior (= zygomaticus major); his superficial portion of the cervical panniculus corresponds to our sphincter colli profundus + superficialis; his deep cervical panniculus corresponds to our sternofacialis, which he describes as an upper limb muscle but, as noted by Jouffroy & Saban 1971, it is in fact a facial muscle that is probably derived from the sphincter colli profundus: e.g. Jouffroy & Saban 1971; Ryan 1986, 1989; this work]
22 (part of platysma sensuLeche 1886)
23 (part of platysma sensuLe Gros Clark 1924 and of notoplatysma sensuLightoller 1934)
24[according to e.g. Gasser 1967 the platysma cervicale (=his nuchal platysma) is present in early developmental stages of modern humans, disappearing in later stages; Aziz 1981 considers that the transversus nuchae found in some humans is a remnant of the platysma cervicale, but Gasser 1967 describes both a platysma cervicale and a transversus nuchae in early human embryos]
25 (seemingly corresponds to pars omoidea sensuSaban 1971)
26 (mixed with platysma cervicale)
27 (platysma myoides superior +’jugalis propatagii’sensuLeche 1886; dorsal sheet of propatagial complex sensuThewissen & Babcock 1991, 1993) [mixed with platysma cervicale; Leche 1886 stated that the propatagial complex of dermopterans has a dorsal muscle formed by the platysma myoides and the ‘jugalis propatagii’ and a ventral muscle; Thewissen & Babcock 1991, 1993, studied the configuration and innervation of these muscles and concluded that the dorsal one is innervated by the facial nerve and the ventral one by cervical spinal nerves; the dorsal and ventral muscles therefore seem to correspond respectively to the platysma myoides and to part of the panniculus carnosus of other mammals]
28[mixed with platysma cervicale]
29 (platysma sensuNetter 2006; tracheoplatysma sensuLightoller 1940a)
30[as described by e.g. Lightoller 1942 in the platypus specimens dissected by us there is a bundle of the platysma that is somewhat similar to the occipitalis of the mammals listed on the right, but this bundle is clearly part of the platysma, i.e. it does not constitute an independent muscle]
31 (cranial part of levator auris longus sensuGreene 1935) [the occipitalis of Rattus is similar to that of Tupaia and Cynocephalus, i.e. it has a medial portion (=occipitalis sensuLightoller 1934) that extends anteriorly to mix with the frontalis and a lateral portion (=cervico-auriculo-occipitalis sensuLightoller 1934) that runs anteroventrolaterally to attach on the posterior surface of the ear; these two portions are deeply mixed posteriorly, attaching to the dorsal region of the neck, just medially to the posterior attachment of the auricularis posterior]
32 (occipitalis + cervico-auriculo-occipitalis sensuLightoller 1934: see 31)
33[Gasser's 1967 developmental study in modern humans indicates that the occipitalis, auricularis posterior and transversus nuchae develop from a same anlage]
34 (caudal part of levator auris longus sensuGreene 1935: see 31)
35[our dissections and comparisons indicate that the platypus has at least some extrinsic muscles of the ear, as suggested by Lightoller 1942; according to Huber 1930a,b, 1931, and Jouffroy & Saban 1971, some of the extrinsic muscles of the ear derive from the platysma while others derive from the sphincter colli profundus]
36[examples of extrinsic, facial muscles of the ear present in therian mammals are the obliquus auriculae, transversus auriculae, helicis, tragicus and/or antitragicus: see e.g. Jouffroy & Saban 1971]
37[although authors such as Adams et al. 1929 suggest that the therian mandibulo-auricularis is a ’preauricular’ muscle and thus derives from the sphincter colli profundus, most researchers consider that it is instead a ’post-auricular’ muscle derived from the platysma (e.g. Huber 1930a,b, 1931; Ryan 1986, 1989); however, a few authors, such as Lightoller 1934 and Jouffroy & Saban 1971, have suggested that the mandibulo-auricularis may in fact be ontogenetically and phylogenetically more related to deeper dorso-median muscles such as the stylohyoideus, digastricus posterior, stapedius and/or jugulohyoideus than to the facial muscles; although we tentatively follow here the most consensual view, we consider that Lightoller's hypothesis should not be completely ruled out, because the mandibulo-auricularis does usually lie deeper to all the other facial muscles and also because its topology, the orientation of its fibers, and its attachments (e.g. on the mandible and/or near the ear region) are in fact similar to those of the deeper dorsomedial hyoid muscles of mammals and to the depressor mandibulae/levator hyoideus of other tetrapods; also, Seiler's 1980 developmental studies of tree-shrews and primates seem to suggest that the mandibulo-auricularis does not develop from the anlages that give rise to most other facial muscles, but instead from a different, deeper anlage]
38[as stated by Lightoller 1934, contrary to lemurs and e.g. Tupaia, in primates as e.g. Tarsius and marmosets the mandibulo-auricularis probably corresponds to a strong sheet connecting the posterior edge of the mandible to the bony external auditory meatus, which might well correspond to the stylo-mandibular ligament of modern humans; such a configuration is found in the colugos dissected, i.e. there is no fleshy muscle mandibulo-auricularis, but instead a strong fascia running from the posterior edge of the mandible to the bony external auditory meatus]
39 (auriculo-mandibularis sensuLightoller 1934)
40[seemingly corresponds to the stylo-mandibular ligament, which is usually present in modern humans: see 38; according to Jouffroy & Saban 1971 it may possibly also correspond to the stylo-auricularis muscle abnormally present in a few modern humans]
41[authors as e.g. Huber 1930a,b, 1931, suggest that the risorius derives from the sphincter colli profundus; our dissections and comparisons support the conclusions of Jouffroy & Saban's 1971 review, i.e. that the risorius derives instead from the platysma myoides; this latter hypothesis is also supported by the developmental data of Gasser 1937: see e.g. his fig. 10]
42 (’géniohyoïdien’+‘hyohyoïdien’sensuMillot & Anthony 1958, which, as is shown in the illustrations of these authors, are effectively deeply mixed]
43 (interhyoideus anterior + interhyoideus posterior sensu Piatt 1938, Bauer 1992, 1997, and Ericsson & Olsson 2004) [as stated by Piekarski & Olsson 2007, recent developmental works indicate that the interhyoideus of sarcopterygians as e.g. Ambystoma might be derived ontogenetically not only from the hyoid region but also possibly from anterior somites]
44 (constrictor colli sensuHerrel et al. 2005)
45 (sphincter colli profundus sensuLightoller, 1942; hyomandibularis sensuEdgeworth, 1935) [Edgeworth, 1935, claimed that none of the mammalian facial muscles are derived from the interhyoideus because all of them are de novo structures; however this view has been abandoned and it is now commonly accepted that the mammalian muscles correspond to the interhyoideus and possibly dorso-medial muscles such as the cervicomandibularis of reptiles such as the lizards Timon: see 20 and also text]
46 (corresponds to Huber's 1930a sphincter colli externus – of platypus – and sphincter colli – of echidna) [corresponds to part of the interhyoideus of non-mammalian tetrapods: e.g. Huber 1930a; Jouffroy & Saban 1971; Lightoller 1940a states that Huber's 1930a sphincter colli superficialis (of e.g. marsupials and rodents) corresponds to his transitus, i.e. to a part of the sphincter colli profundus that passes superficial to the platysma but that originally was deep to it: Lightoller claims that the rest of the sphincter colli profundus (i.e. everything except the transitus) is absent in all primates, and that it is thus this transitus that gives the tracheo-platysma of primates; however, our dissections and comparisons strongly indicate that the configuration of the platysma of primates such as lemurs is in fact similar to that found in e.g. colugos and tree-shrews, i.e. these latter mammals have both a platysma cervicale and a platysma myoides, although these two latter muscles are blended; in primates such as modern humans, the platysma cervicale is usually missing, i.e. the platysma sensuNetter 2006 corresponds to the platysma myoides of other mammals; if we accept, as it is nowadays commonly accepted, that the sphincter colli of mammals derives from the interhyoideus of other tetrapods, it makes sense to suppose that plesiomorphically the sphincter colli was a superficial muscle, as is the non-mammalian interhyoideus, and not a deep muscle: see text]
47 (transitus sensuLightoller 1942) [as explained by e.g. Lightoller 1940a, 1942, although much reduced, in rodents such as rats the sphincter colli does have a component that is superficial to the platysma – i.e. a sphincter colli superficialis]
48[Jouffroy & Saban 1971, p. 484, state that colugos have a sphincter colli superficialis, but as they explain in their p. 496, this is because they consider that the ventral sheet of the propatagial muscle complex of colugos probably corresponds to the sphincter colli superficialis of other mammals; Thewissen & Badcock 1991, 1993, have however shown that this ventral sheet is in fact innervated by cervical spinal nerves and not by the facial nerve, as is the sphincter colli superficialis; moreover, as shown in fig. 1 of these latter authors, the position and the orientation of the fibers of that ventral sheet are not similar to those of the sphincter colli superficialis of other mammals (e.g. in a lateral view it appears deep, not superficial, to the platysma); our dissections indicate that colugos do not have a fleshy, distinct muscle sphincter colli superficialis]
49 (seems to correspond to the occipito-cervicalis sensuLightoller 1934, and might correspond to the cervico-mandibularis sensuLe Gros Clark 1926, which was originally described as part of the platysma of Ptilocercus but seems rather to correspond to the sphincter colli superficialis of Tupaia and other mammals)
50[it is commonly accepted that primates such as modern humans and chimpanzees do not have a sphincter colli superficialis, but according to Burrows et al. 2006 this muscle may be found in some chimpanzees and perhaps even in some modern humans; in the modern human cadavers we dissected the sphincter colli superficialis was not present as an independent muscle]
51[absent as an independent muscle in the platypus, although part of it might have given rise to deep facial muscles such as the orbicularis oris, orbicularis oculi, mentalis and/or naso-maxillo-labialis (which clearly seem to correspond to the muscles that are designated by the same names in other mammals) and possibly to the 'sphincter bursae buccalis’sensu Huber 1930a (which, contrary to what was stated by Huber 1930a, seems to correspond to the ’buccinatorius’ of the echidna and to the buccinatorius of other mammals); in the echidna part of the sphincter colli passes deep to other facial muscles, forming the sphincter colli profundus: e.g. Lightoller 1942; Jouffroy & Saban 1971]
52 (superficial portion of cervical platysma sensuGreene 1935; sphincter colli profundus +’primitive sphincter colli’ of fig. 6 of Huber 1930a; transitus sensuLightoller 1940a) [deeply mixed with the sphincter colli superficialis]
53[absent according to Jouffroy & Saban 1971, but this muscle is clearly present in the specimens dissected: dorsally its runs deep to the platysma myoides while ventrally it meets its counterpart in the ventral midline of the head]
54 (deep cervical panniculus sensuGreene 1935: see 21)
55[its position and the orientation of its fibers are similar to those of the interscutularis of non-monotreme mammals such as rats; Lightoller 1940a seems to support the homology of these muscles, because he states that there is a cervicalis transversus in rodents; however, according to Jouffroy & Saban 1971, the interscutularis is derived from the pars intermedia of the sphincter colli profundus, while the cervicalis transversus is derived from the pars cervicalis of this latter muscle]
56[our dissections and comparisons support Jouffroy & Saban's 1971 hypothesis, i.e.: 1) the zygomaticus major and minor are absent in mammals such as monotremes; 2) in placentals, the zygomaticus is plesiomorphically attached to the zygomatic arch, but in some cases it extends posteriorly to attach to the ear (that is why it is sometimes named auriculolabialis); 3) in a few mammals, such as some ungulates, pinnipedes, bats, rodents, tree-shrews and primates, the zygomaticus is divided into superficial (= auriculolabialis inferior and zygomaticus major sensuJouffroy & Saban 1971) and deep (= auriculolabialis superior and zygomaticus minor sensuJouffroy & Saban 1971) portions, the former originating ventrally and/or posteriorly to the latter, thus usually lying nearer the ear and being more associated with the platysma (that is why some authors argue that it might derive from the platysma, although its innervation seems to indicate the contrary); Jouffroy & Saban 1971 explicitly state that these superficial and deep portions correspond very likely to the zygomaticus major and minor of modern humans, respectively; 4) according to them, in mammals such as tree-shrews and primates as lemurs the zygomatic muscles, and particularly the zygomaticus major, often extends posteriorly in order to attach to the ear (Fig. 12), but this ‘trend’ is reverted in higher primates: for example, in modern humans both the zygomaticus major and zygomaticus minor usually originate relatively far from the ear, although in a few cases at least one of these muscles might originate on the ear region]
57 (part or the totality of auriculolabialis sensuGreene 1935; zygomatico-labialis superficialis and or auriculolabialis inferior sensuJouffroy & Saban 1971) [our dissections indicate that the ’auriculolabialis’ of the Norwegian rat is deeply mixed with the platysma; does this mean that it is really part of and/or derived from the platysma? Probably not, because as stated by Greene 1935 in other rats and other rodents this ’auriculolabialis’ is much more distinct from the platysma, being seemingly a derivative of the sphincter colli profundus; however, it is possible that some of the mammalian structures that are designated as ’zygomaticus major and minor’ and/or ’auriculolabialis inferior and superior’ in the literature are really part of and/or derive from the platysma, i.e. that they are not really homologous to the zygomaticus major and minor sensu this work: e.g. Boas & Paulli 1908; Huber 1930a; Edgeworth 1935; Jouffroy & Saban 1971; it cannot be completely ruled out, however, that at least in some cases the ‘zygomaticus major’ and/or ‘auriculolabialis inferior’ derive from the platysma, while the ‘zygomaticus minor’ and/or ‘auriculolabialis superior’ derive from e.g. the orbicularis oculi]
58[in Huber's 1930a fig. 27 of the ’primate ground plan of superficial facial musculature’, he suggests that the auriculabialis inferior (= zygomaticus major) derives from the platysma, but at least in the case of colugos the former muscle is well distinguished from the latter, because these muscles are in fact perpendicular to each other; there is a significant difference between Cynocephalus, Lemur and Tupaia: in Cynocephalus the auriculolabialis inferior (= zygomaticus major) is superficial to the platysma cervicale; in Lemur these two muscles lie in the same plane; in Tupaia the auricularis inferior is deep to the platysma cervicale: e.g. Lightoller 1934; this work]
59 (auriculolabialis inferior or zygomatico-labialis sensuJouffroy & Saban 1971, Le Gros Clark 1926, and Lightoller 1934)
60[Gasser's 1967 developmental study indicate that the zygomaticus major and zygomaticus minor of modern humans derive from his ’infraorbital lamina’, and not from his ‘mandibular lamina’, i.e. they seem to be ontogenetically more related with the facial muscles of the orbit region than with those of the mouth region; interestingly, in earlier stages of human development these two muscles are more separated from each other than in later stages, i.e. in this respect the configuration seen in those early stages is more similar to that seen in adult mammals such as colugos, tree-shrews and lower primates: see e.g. Fig. 12]
61 (zygomaticus sensuGreene 1935; zygomatico-labialis profundus and or auriculolabialis superior sensuouffroy & Saban 1971)
62 (auriculolabialis superior sensuJouffroy & Saban 1971, Le Gros Clark 1926, and Lightoller 1934)
63 (the frontalis sensuGreene 1935 corresponds to the orbito-temporo-auricularis sensuEdgeworth 1935)
64[our dissections and comparisons indicate that the frontalis and auriculo-orbitalis of Cynocephalus and Tupaia and the frontalis, temporoparietalis and auricularis anterior of modern humans, correspond to the orbito-temporo-auricularis of mammals such as rats]
65[this muscle usually runs from the auricular region to the orbital region, being inferior and/or deep to the frontalis: e.g. Fig. 12]
66 (auriculo-orbitalis or orbito-auricularis sensuLightoller 1934; it might correspond to Tupaia's attrahens aurem sensuLe Gros Clark 1924, and/or to the Ptilocercus’ scutularis + portio transiens sensuLe Gros Clark 1926)
67[according to Jouffroy & Saban 1971, this muscle is related to, but different from, the auricularis superior; they state that it corresponds to the temporal part of the frontalis, which is also named epicranio-temporal or orbito-temporalis, and which has a longitudinal orientation and covers the temporal aponeurosis, being often fused in primates with the auriculares anterior and superior and also to part of the galea aponeurotica]
68[contrary to Ptilocercus, Tupaia seems to only have an auriculo-orbitalis sensuLightoller 1934, i.e. it does not have a separate temporoparietalis and a separate auricularis anterior: see 66]
69 (auricularis anterior superior of e.g. fig. 409 of Jouffroy & Saban 1971)
70[there is a very thin group of fibers attaching to the medial margin of the posterior portion of the orbicularis oculi, anteriorly, and to the dorsal surface of the zygomatic arch, posteriorly; this thin group of fibers lies deep to most fibers of the orbicularis oculi and auriculo-orbitalis, being in fact deeply mixed with the temporal fascia covering the temporalis in lateral view; however, our dissections indicate that there are in fact some fleshy fibers, which thus should be considered a zygomatico-orbicularis sensuLe Gros Clark 1924, 1926, even if this is a poorly developed muscle; the lemur shown in fig. 4 of Lightoller 1934 also seems to have such a group of fleshy fibers]
71[Lightoller 1934 states that in Tupaia javanica there is a group of fibers running from the region lying posterodorsally to the eye to the dorsal margin of the zygomatic arch, but that it cannot correspond to the zygomatico-orbicularis sensuLe Gros Clark 1924 because it lies deep to the orbicularis oculi; however, Le Gros Clark 1924 stated that his zygomatico-orbicularis lies deep to at least some fibers of the orbicularis oculi; the group of fibers described by Lightoller thus seems to correspond to the zygomatico-orbicularis sensu Le Gros Clark]
72[the depressor supercillii and corrugator supercilii are seemingly derived from the orbicularis oris]
73 (superciliaris sensuJouffroy & Saban 1971)
74[Lightoller 1942 and Saban 1971 state that deep to the cranial (anterior) portion of the orbicularis oculi of the echidna lies a small naso-labialis; according to Lightoller 1942 in the platypus there is a somewhat similar structure, but it is not as differentiated from the other facial musculature as in the echidna; in the platypus dissected by us the naso-labialis does seem to be an independent muscle, being in fact very similar to the naso-labialis of the echidna - see e.g. fig. 4 of Lightoller 1942, which suggests that the naso-labialis derives from a part of the sphincter colli superficialis (transitus sensu Lightoller): Huber 1930a; Jouffroy & Saban 1971]
75 (levator labii superioris sensuParsons 1898 and Greene 1935; pars jugularis of superficial maxillo-naso-labialis sensuLightoller 1940b)
76 (levator labii superioris sensuLe Gros Clark 1924) [not described by Le Gros Clark, 1926 but his fig. 49 seems to suggest that it may also be present in Ptilocercus]
77[the levator labii superioris and levator labii superioris alaeque nasi clearly seem to correspond to the naso-labialis of the other therian mammals listed on this Table; Gasser's 1967 developmental study suggests that at least in modern humans these two muscles appear ontogenetically in the orbital region]
78 (nasolabialis superficialis sensuRyan 1989) [not described by Greene 1935 but seems to be present in the rats dissected by us; see also e.g. Ryan 1989]
79 (the buccinatorius sensuLightoller 1942 corresponds to the sphincter bursae buccalis sensu Huber 1930a) [according to Huber 1930a there is no buccinatorius in the platypus, and the ‘buccinatorius’ of echidna may well not be homologous with that of other mammals, because it may well be derived from the platysma and not from the sphincter colli profundus; however, as noted in later works as e.g. Lightoller 1942 and Jouffroy & Saban 1971, the ‘sphincter bursae buccalis’ of platypus might well correspond to the ‘buccinatorius’ of echidna and/or to the buccinatorius of other mammals]
80[not described by Greene 1935 but is clearly present in the rats dissected by us, being in fact subdivided into various sections; see also e.g. Ryan 1989]
81[not described by Le Gros Clark 1924, but it is clearly present in the Tupaia specimens we dissected; it is also present in Ptilocercus, see e.g. Le Gros Clark 1926]
82 (dilatator naris sensuGreene 1935 and Peterka 1936) [we prefer to use the name dilatator nasi because the name dilatator naris is often used to designate the pars alaris of the nasalis: see e.g. Jouffroy & Saban 1971]
83 (seemingly incorporates the maxillo-labialis and naso-labiais profundus sensuJouffroy & Saban 1971 and Ryan 1989) [not described by Greene 1935, but it is clearly present in the rats dissected by us; see also e.g. Ryan 1989]
84 (maxillo-nasalis sensuJouffroy & Saban 1971; it might correspond to the dilator naris, zygomatici and/or erector vibrissae sensuLe Gros Clark 1924, and thus might possibly be included in the orbicularis oculi (sensue Gros Clark 1926) of Ptilocercus)
85[the nasalis and depressor septi nasi of modern humans seem to correspond to the maxillo-naso-labialis of e.g. rats, colugos and tree-shrews: see e.g. Jouffroy & Saban 1971]
86 (levator anguli oris or caninus sensuLightoller 1934; bucco-naso-labialis sensuRyan 1986; buccinatorius sensuGreene 1935, and Bryant 1945; pars profunda of maxillo-naso-labialis sensuLightoller 1940b) [we use the name levator anguli facialis in order to distinguish this muscle from the levator anguli oris mandibularis of some non-mammalian tetrapods, which is a mandibular, and not a hyoid, muscle: see Table 1]
87 (levator anguli oris sensuLe Gros Clark 1926; incisivus superior + caninus sensuLightoller 1934) [as noted by Lightoller 1934, it is deeply mixed with the orbicularis oris]
88[Gasser's 1967 study of human development supports the claim that the levator anguli oris facialis, orbicularis oris, depressor labii inferioris, depressor anguli oris and mentalis have a common ontogenetic origin, being derived from his ‘mandibular lamina’; see also e.g. Sullivan & Osgood 1927, and Jouffroy & Saban 1971]
89 (plicae anguli oris sensu Huber 1930a)
90[present in the platypus, but seemingly not in echidna, according to e.g. Lightoller 1942 and Saban 1971]
91 (labiorum profundi inferioris sensuLightoller 1934)