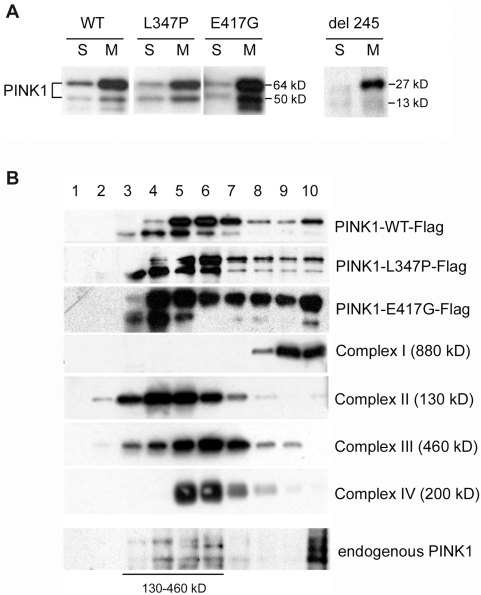
Figure 4. Recombinant and endogenous PINK1 are associated with protein complexes in mitochondria.
In all experiments, adenoviruses of PINK1-Flag, L347P-PINK1-Flag, E417G-PINK1-Flag or Del 245 PINK1-Flag were infected into SH-SY5Y cells. A) Western blot analysis of PINK1 sub-cellular distributions with anti-Flag Ab. The two forms of PINK1 (a 64 kD full length protein and a truncated form of 50 kD, presumably a proteolytic product) are present in mitochondria and cytosol. The L347P, E417G or Del 245 mutant PINK1 did not affect this distribution. S: cytosolic fraction; M: mitochondrial fraction. B) PINK1 is associated with protein complexes. Mitochondrial proteins were sub-fractionated by 15% to 35% discontinuous sucrose gradient, from which fractions 1–10 were collected from top (lighter proteins) to bottom (heavier proteins or complexes). They were subjected to SDS-PAGE, and Western analyses with anti-Flag Ab for PINK1 (the top three panels); anti-39 kD protein Ab for complex I (4th panel); anti-70 kD protein Ab for complex II (5th panel); anti-core 2 Ab for complex III (6th panel); and anti-cox I Ab for complex IV (7th panel). No PINK1 was observed in lane 1 and 2, the fractions that contained proteins of the sizes for monomeric PINK1. Instead, PINK1 was associated with protein complexes ranging from 130–900 kD, which co-migrated with ETC complexes. The L347P and E417G mutations did not affect the PINK1 association and distribution of these complexes. More importantly, anti-human PINK1antibodies (Novus) detected endogenous PINK1 in SH-SY5Y cells with similar distribution along the sucrose gradient (bottom panel).