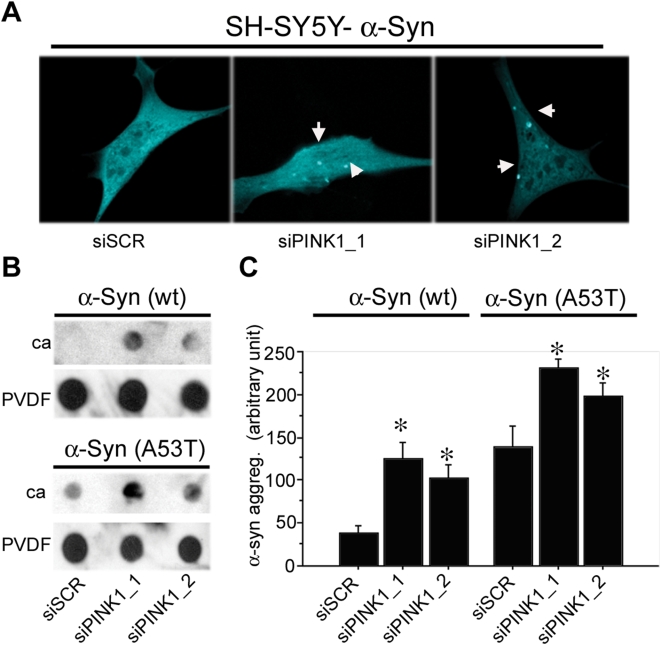
Figure 9. Loss of PINK1 function leads to α-synuclein aggregation.
Knockdown of PINK1 by siRNAs in cell lines expressing wild type α-synuclein-mCFP or A53T α-synuclein-mCFP leads to the accumulation and aggregation of α-synuclein as measured by confocal microscopy or filter trap assay. A) Representative confocal images of SH-SY5Y cells co-transfected with α -synuclein-mCFP and siRNA. The results indicate that loss of PINK1 leads to the formation of mCFP-positive inclusions. B) Dot blot filter trap assay captured SDS-insoluble inclusions formed by loss of PINK1 function. Two different siRNA with sequences against PINK1 (siPINK1-1 and siPINK1-2) were transfected into stable HeLa cell lines expressing wt α-synuclein or A53T α-synclein. The lysate was collected and filtered through two filters, with the cellulose acetate (ca) membrane on the top and the PVDF membrane at the bottom. The ca membrane trapped the insoluble aggregates, whereas PVDF membrane caught the soluble protein. The results indicate that reduction of PINK1 by either siRNA leads to increased accumulation of SDS insoluble α-synuclein compared with the scrambled siSCR control when filtered through ca membrane. PVDF membrane was blotted with actin as loading control. C) Quantification of filter trap assay using densitometry analysis via NIH Image. Each bar represents 4 samples. siRNA-mediated knockdown of PINK1 leads to a significant increase of SDS-insoluble α-synuclein (*: p<0.05). ANOVA analysis reveals a significant effect of α-synuclein (F(1,18) = 56.482; p<0.001) and siRNA (F(2,18) = 15.559; p<0.001), but no significant interaction between the two variables (F(2,18) = 55.413; p = 0.949), thus indicating that PINK1 knockdown has a similar effect on both forms of α-synuclein.