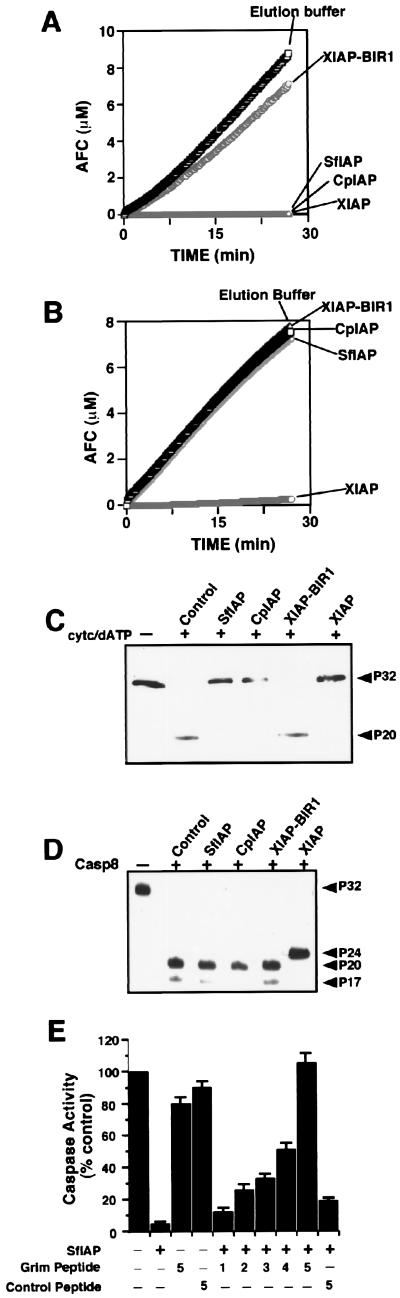
Figure 3.
SfIAP and CpIAP suppress Cyt c- but not caspase-8-induced activation of effector caspases. Recombinant SfIAP or CpIAP (2 μM) was added to cytosolic extracts (10 mg/ml) from HEK293 cells concurrently with the addition of 1 μM Cyt c/10 mM dATP (A) or 100 ng/ml active caspase-8 (B). After incubation at 30°C for 10 min, aliquots were withdrawn and assayed for caspase activity, continuously monitoring release of AFC from Ac-DEVD-AFC substrate (100 μM) beginning from the time of substrate addition. SD from 10 replicates was smaller than the points on the graphs. (C and D) Aliquots of cell extracts from the experiments performed above were analyzed by SDS/PAGE–immunoblotting by using anticaspase-3 antiserum. Arrowheads indicate the pro- and processed forms of caspase-3. In cell extracts activated by Cyt c or active caspase-8, ≈32-kDa procaspase-3 was processed to yield ≈17- to 20-kDa forms of the large subunit, indicative of active caspase-3 (the ≈12-kDa subunit of caspase-3 is undetectable with this anticaspase-3 antibody). Recombinant SfIAP and CpIAP suppressed the processing of procaspase-3 in Cyt c-treated (C) but not in caspase-8-treated (D) extracts, whereas XIAP interrupted processing at an intermediate step (≈24-kDa band), as demonstrated previously (10, 11). (E) Grim peptide or control peptide (2–10 μM) was added with or without recombinant SfIAP (2 μM) protein to HEK293 cell lysates concurrently with the addition of Cyt c/dATP. Lysates were incubated at 30°C for 10 min, and aliquots then were withdrawn and assayed for DEVD-cleaving caspase activity as above, measuring rates from the linear portion of enzyme progress curves.Data are presented as a percentage relative to control reactions in which Cyt c/dATP were alone. Numbers below diagram indicate molar ratio of peptides relative to SfIAP protein.