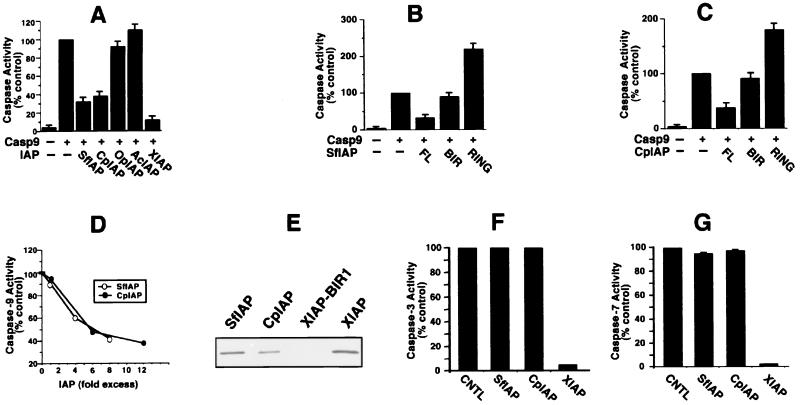
Figure 4.
SfIAP and CpIAP directly suppress caspase-9 but not caspase-3 or caspase-7. Caspase-9 expression plasmids were cotransfected into 293 cells with plasmids encoding various full-length IAPs (A) or fragments of SfIAP (B) comprising only the BIR domains (residues 1–323) or RING domain (residues 324–377) or fragments of CpIAP containing only BIR (residues 1–220) or RING (residues 221–275) domains (C). After 24–36 hr, lysates were prepared and assayed for DEVD-cleaving caspase activity, as above. Data are presented as a percentage relative to caspase activity of cells transfected with caspase-9 alone (mean ± SD; n = 3). (D) Recombinant active caspase-9 was added at 0.2 μM because of its lower specific activity and incubated at 37°C with Ac-LEHD-AFC substrate (100 μM) in the presence or absence of various concentrations (0.2–1.6 μM) of SfIAP (○) or (0.2–2.4 μM) of CpIAP (●). AFC release was measured continuously, and data are expressed as a percentage relative to control reactions lacking IAPs, using rates determined from the linear portion of enzyme progress curves. Various control GST-fusion proteins had no inhibiting effect (not shown). (E) [35S]Caspase-9 in reticulocyte lysate was incubated with GST-fusion proteins immobilized on glutathione-Sepharose: GST-SfIAP, GST-CpIAP, GST-XIAP-BIR1, and GST-XIAP. (F) Recombinant active caspase-3 (2.6 nM) was incubated at 37°C with Ac-DEVD-AFC substrate (100 μM) in the presence or absence of 0.05 μM GST-XIAP, 0.5 μM GST-SfIAP (200-fold molar excess relative to caspases), or 0.5 μM GST-CpIAP (≈200-fold molar excess). AFC release was measured as above. (G) Caspase-7 (7 nM) was incubated at 37°C with Ac-DEVD-AFC substrate (100 μM) in the presence or absence of 0.14 μM GST-XIAP, 0.7 μM GST-SfIAP (100-fold molar excess relative to caspases), or 0.6 μM GST-CpIAP (≈100-fold molar excess). AFC release was measured as above.