Abstract
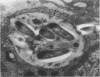
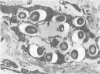
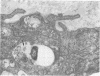
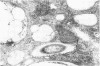
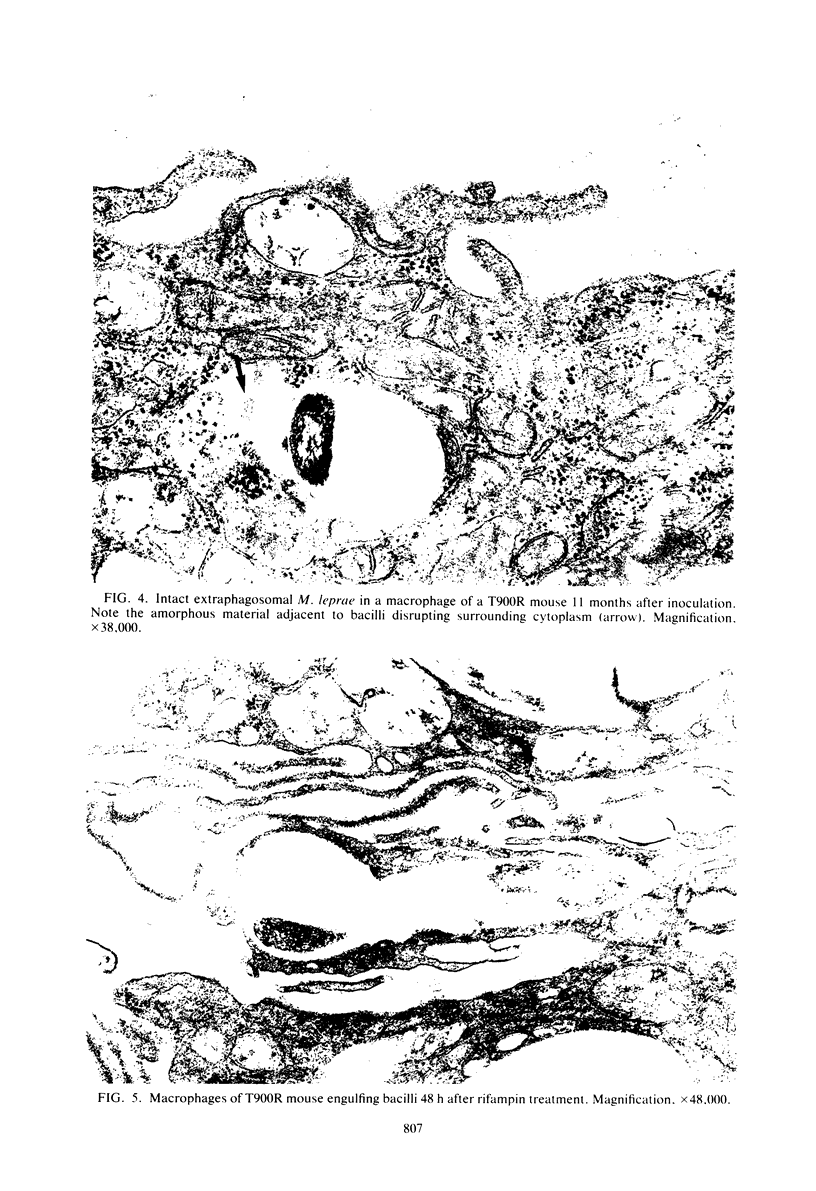
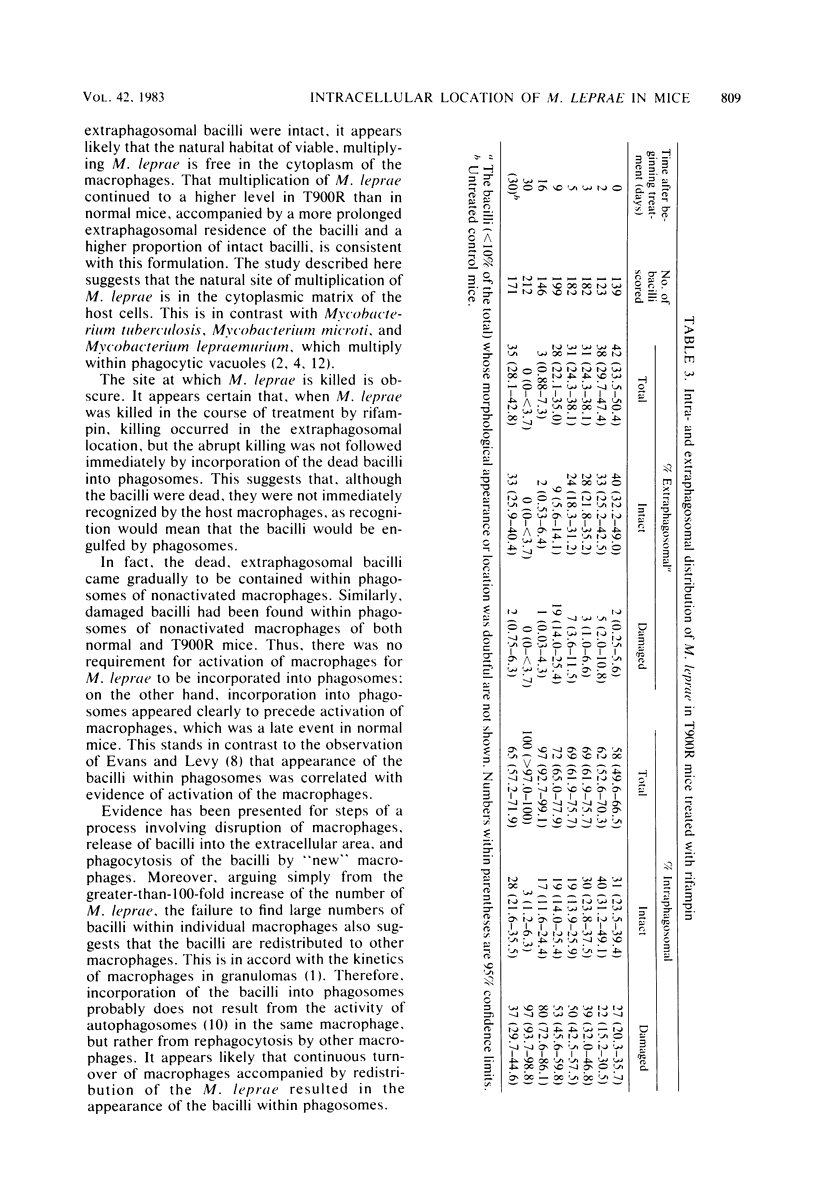
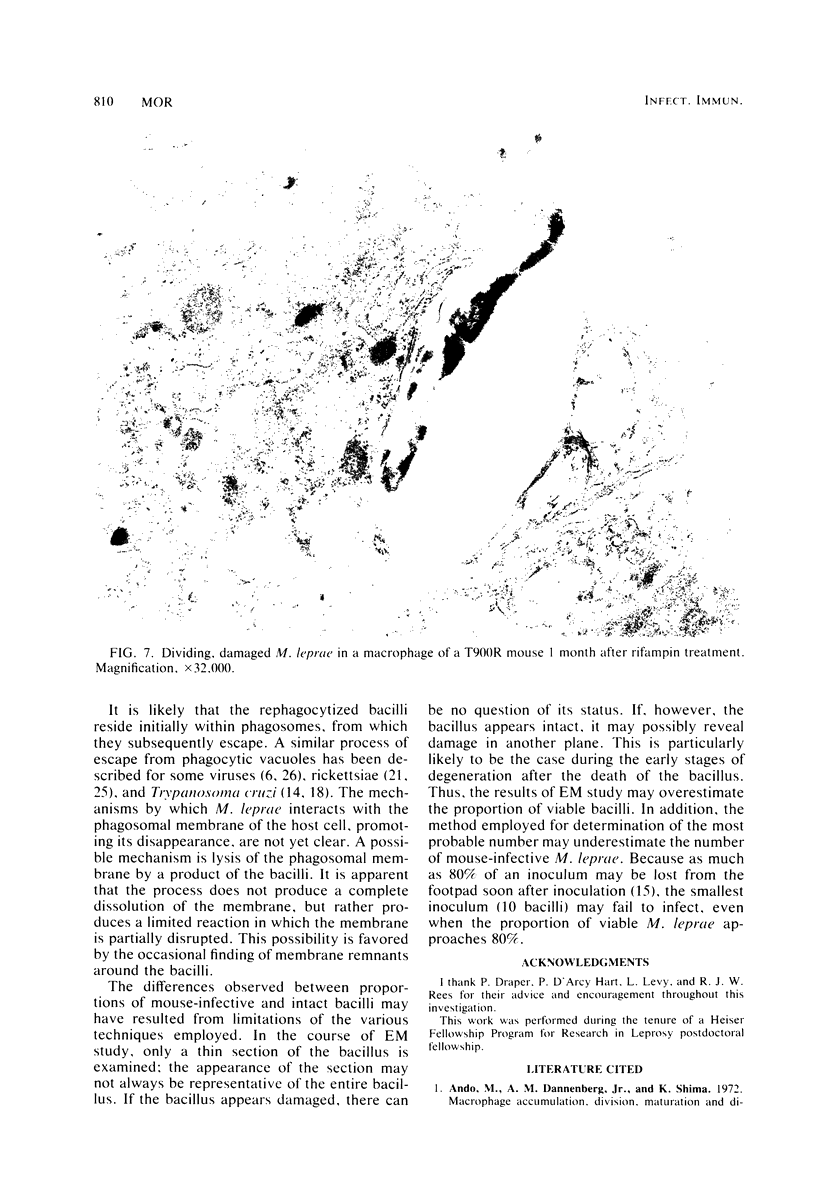
Soon after more than 10(6) Mycobacterium leprae, freshly harvested from armadillo liver or harvested and 60CO irradiated, were inoculated into the hind footpads of either normal or thymectomized and irradiated (T900R) mice, the organisms were found to reside within phagosomes of polymorphonuclear and mononuclear cells. On the other hand, 7 and 8 months after 10(4) freshly harvested M. leprae were inoculated into the footpads of normal or T900R mice and the organisms had multiplied to their maximum in the normal mice, many organisms, largely intact by electron-microscopic criteria, were found to reside free in the cytoplasm of the footpad macrophages, whereas damaged organisms were contained within phagosomes. After 11 months, many intact organisms were found to lie free in the cytoplasm of the macrophages of T900R mice, whereas only damaged intraphagosomal M. leprae cells were observed in the macrophages of normal mice. Finally, a remarkably large proportion of damaged extraphagosomal M. leprae was found in T900R mice administered rifampin for 2 days in a bactericidal dosage. It appears that M. leprae multiplies free in the cytoplasm of the footpad macrophages of infected mice, whereas the M. leprae cells resident within the phagosomes of the macrophages are dead. As the result of treatment with rifampin, the organisms appeared to have been killed in their extraphagosomal location, only afterwards being incorporated into phagosomes. However, the intracellular site in which M. leprae is killed in the course of an effective immune response remains unclear.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M., Dannenberg A. M., Jr, Shima K. Macrophage accumulation, division, maturation and digestive and microbicidal capacities in tuberculous lesions. II. Rate at which mononuclear cells enter and divide in primary BCG lesions and those of reinfection. J Immunol. 1972 Jul;109(1):8–19. [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddingius J. Ultrastructural changes in blood vessels of peripheral nerves in leprosy neuropathy. II. Borderline, borderline-lepromatous and lepromatous leprosy patients. Acta Neuropathol. 1977 Sep 26;40(1):21–39. doi: 10.1007/BF00688570. [DOI] [PubMed] [Google Scholar]
- Brown C. A., Draper P. An electron-microscope study of rat fibroblasts infected with Mycobacterium lepraemurium. J Pathol. 1970 Sep;102(1):21–26. doi: 10.1002/path.1711020105. [DOI] [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri M. M., Weddell A. G., Rees R. J. Infection of murine striated muscle with Mycobacterium leprae: a study by light and electron microscopy. J Pathol. 1972 Feb;106(2):73–80. doi: 10.1002/path.1711060203. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Levy L. Ultrastructural changes in cells of the mouse footpad infected with Mycobacterium leprae. Infect Immun. 1972 Feb;5(2):238–247. doi: 10.1128/iai.5.2.238-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Newton H. E., Levy L. Early response of mouse foot pads to Mycobacterium laprae. Infect Immun. 1973 Jan;7(1):76–85. doi: 10.1128/iai.7.1.76-85.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G., Cohn Z. A. Autophagic vacuoles produced in vitro. I. Studies on cultured macrophages exposed to chloroquine. J Cell Biol. 1968 Aug;38(2):377–391. doi: 10.1083/jcb.38.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HART P. D., REES R. J. Effect of macrocyclon in acute and chronic pulmonary tuberculous infection in mice as shown by viable and total bacterial counts. Br J Exp Pathol. 1960 Aug;41:414–421. [PMC free article] [PubMed] [Google Scholar]
- Halvorson H. O., Ziegler N. R. Application of Statistics to Problems in Bacteriology: I. A Means of Determining Bacterial Population by the Dilution Method. J Bacteriol. 1933 Feb;25(2):101–121. doi: 10.1128/jb.25.2.101-121.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress Y., Bloom B. R., Wittner M., Rowen A., Tanowitz H. Resistance of Trypanosoma cruzi to killing by macrophages. Nature. 1975 Oct 2;257(5525):394–396. doi: 10.1038/257394a0. [DOI] [PubMed] [Google Scholar]
- Levy L., Moon N., Murray L. P., O'Neill S. M., Gustafson L. E., Evans M. J. Studies of the mouse foot pad technic for cultivation of Mycobacterium leprae. 1. Fate of inoculated organisms. Int J Lepr Other Mycobact Dis. 1974 Apr-Jun;42(2):165–173. [PubMed] [Google Scholar]
- Levy L., Ng H., Evans M. J., Krahenbuhl J. L. Susceptibility of thymectomized and irradiated mice to challenge with several organisms and the effect of dapsone on infection with Mycobacterium leprae. Infect Immun. 1975 May;11(5):1122–1132. doi: 10.1128/iai.11.5.1122-1132.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L., Shepard C. C., Fasal P. The bactericidal effect of rifampicin on M. leprae in man: a) single doses of 600, 900 and 1200 mg; and b) daily doses of 300 mg. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):183–187. [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES R. J. LIMITED MULTIPLICATION OF ACID-FAST BACILLI IN THE FOOT-PADS OF MICE INOCULATED WITH MYCOBACTERIUM LEPRAE. Br J Exp Pathol. 1964 Apr;45:207–218. [PMC free article] [PubMed] [Google Scholar]
- Rees R. J. Enhanced susceptibility of thymectomized and irradiated mice to infection with Mycobacterium leprae. Nature. 1966 Aug 6;211(5049):657–658. doi: 10.1038/211657a0. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., Levy L., Fasal P. Rapid bactericidal effect of rifampin on Mycobacterium leprae. Am J Trop Med Hyg. 1972 Jul;21(4):446–449. doi: 10.4269/ajtmh.1972.21.446. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., Van Landingham M., Redus M. A. Kinetic testing of drugs against Mycobacterium leprae in mice. Activity of cephaloridine, rifampin, streptovaricin, vadrine, and viomycin. Am J Trop Med Hyg. 1971 Jul;20(4):616–620. doi: 10.4269/ajtmh.1971.20.616. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr In vitro studies of rickettsia-host cell interactions: ultrastructural changes induced by Rickettsia rickettsii infection of chicken embryo fibroblasts. Infect Immun. 1979 Nov;26(2):714–727. doi: 10.1128/iai.26.2.714-727.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. Macrophages and viral immunity. Semin Hematol. 1970 Apr;7(2):185–214. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- WATERS M. F., REES R. J. Changes in the morphology of Mycobacterium leprae in patients under treatment. Int J Lepr. 1962 Jul-Sep;30:266–277. [PubMed] [Google Scholar]
- Welch T. M., Gelber R. H., Murray L. P., Ng H., O'Neill S. M., Levy L. Viability of Mycobacterium leprae after multiplication in mice. Infect Immun. 1980 Nov;30(2):325–328. doi: 10.1128/iai.30.2.325-328.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]