Abstract
Purpose
Partnership for Health (PFH) was found to increase smoking cessation among smokers in the Childhood Cancer Survivors Study (CCSS) at the 8- and 12-month postbaseline follow-up. This report provides outcomes at 2 to 6 years postbaseline; the primary outcome is a four-category smoking status variable (quit at all follow-ups, quit at final follow-up only, smoker at all follow-ups, and smoker at final follow-up only); quit attempts among those who reported smoking at the final follow-up is a secondary outcome.
Methods
PFH was a randomized control trial with two conditions, peer phone counseling (PC) and self-help (SH), that involved smokers (n = 796) enrolled in the CCSS cohort.
Results
Long-term quit rates were higher in PC versus SH participants. Long-term smoking cessation outcomes were lower among those who were nicotine dependent, of lower educational levels, and among men, and were higher among those who used nicotine replacement therapy and who had higher levels of situational self-efficacy. There were no significant differences in relapse rates between conditions or in quit attempts among continued smokers.
Conclusion
Cessation rates continue to be significantly higher among participants in the PC condition versus SH, although the differences were not large. This article highlights differences in long-term engagement with smoking cessation among those who received the intervention.
INTRODUCTION
Mortality rates associated with childhood cancer have been dramatically reduced over the past several decades, but recent research has identified significant late effects across multiple organ systems.1-10 Efforts to reduce preventable risk factors, such as smoking, should be a priority in this population.3,11-16 More than 17% of childhood cancer survivors report active smoking.16 Partnership for Health (PFH), a randomized control trial using a peer-delivered telephone counseling (PC) program, led to a doubling of quit rates versus a self-help condition (SH). This article reports on the long-term smoking outcomes of PFH participants and describes the long-term experiences of smokers engaged in cessation efforts.
METHODS
The Childhood Cancer Survivor Study (CCSS) is a multisite study of the late effects of childhood cancer treatment.17-20 Smokers identified on the CCSS baseline questionnaire and who met the following eligibility criteria were invited to participate in PFH: ≥ 18 years of age; not currently in treatment for cancer; mentally able to provide informed consent; and the ability to read and speak English. Study methods are reported elsewhere.20 This study was approved by the human subjects committees at the participating institutions, and followed standard ethical practices.
Sample
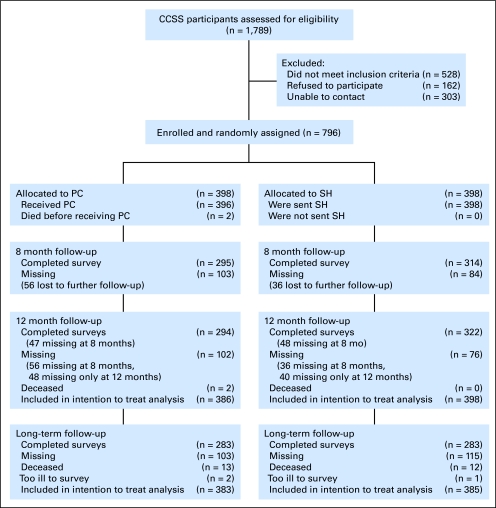
We attempted to assess eligibility for PFH among 1,789 potentially eligible CCSS participants. Among these, 796 enrolled in PFH (recruited May 1999 to July 2000), representing 63% of the overall sample and 83% of those we could reach and determine eligibility.20 A CCSS follow-up survey, which assessed current smoking status, was conducted in waves beginning in 2002, and was completed by 566 PFH participants (Fig 1). Most participants (69%) completed the long-term (LT) assessment between 2 and 4 years after enrollment in PFH (range, 1.8 to 5.7 years). There were no significant differences by PFH condition in time to follow-up.
Fig 1.
Recruitment and retention rates (CONSORT statement).
Intervention Conditions
SH and PC intervention.
SH participants received a letter from study physicians about smoking and late effects and a cessation manual.21
PC intervention participants were assigned a peer counselor (also a survivor) who provided up to six telephone counseling calls within a 7-month period. Written materials tailored on the interaction of smoking with cancer type and treatment and psychosocial variables were provided.
Measures
The primary outcome variable is a four-category measure of smoking status based on 7-day point-prevalence smoking status at the end of the PFH intervention and LT follow-up among the entire sample, including: continuous smoker (smoker at each assessment), relapser (nonsmoker at the end of PFH and a smoker at LT follow-up), delayed quitter (smoker at the end of PFH and nonsmoker at LT follow-up), and continuous quitter (nonsmoker at both time points). Quit attempts were evaluated among continuous smokers and relapsers; thus, a four-category variable created (0, 1 to 2, 2 to 5, and 6+ attempts). Exploratory analyses used a three- category variable labeled time to follow-up to take into consideration the varying length of follow-up: 1.8 to fewer than 3 years, 3 to 4 years, and 4+ years. We also used 7-day point prevalence smoking status at LT follow-up in our exploratory analyses.
Predictor variables were examined in relationship to primary outcomes. Nicotine dependence was measured as time from waking to first cigarette.22 Self-efficacy was defined using single-item measures of confidence in one's ability to quit smoking in at 1 and 6 months, and confidence in not smoking in a variety of situations.23 Readiness to quit smoking was assessed using the stages of change algorithm.24 Depressed mood was assessed using a single item reflecting feelings of being downhearted and blue in the previous 2 weeks.25-27
Demographics assessed included: age, sex, race, ethnicity, socioeconomic status, education, employment history, marital status, and medical history. Participation was solicited by mail sent from a single coordinating center. There were no differences in baseline variables across cancer centers, and no relationship between cancer center and outcomes of interest.
Data Analysis
All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). The outcome evaluation began with univariate descriptive analyses. Participants with missing data on smoking rate at each time point were conservatively assumed to be smokers, as is standard practice.28 Two-way analyses using χ2 statistics for categoric variables and analysis of variance for continuous variables were used to assess the relationships between smoking cessation and the predictor variables. These variables were a subset of all those collected and were chosen based on variables that the literature has indicated are associated with smoking cessation outcomes. A polytomous logistic regression model predicting cessation was developed using all variables from the bivariate analyses with statistical significance at the P = .10 level. The referent category was continuous quitter. A final parsimonious model included only significant variables and effect modifiers. All potential independent variables were tested for mediation.29 Tests for moderating effects (interaction) between significant variables and intervention group were conducted. Model assessment was conducted using log-likelihood goodness of fit statistics. Exploratory polytomous logistic models were created for quit attempts, with referent category being the 6+ attempt group.
Exploratory logistic regression models of smoking status at LT follow-up were examined, comparing the models across the three categories of time to follow-up. An exploratory subanalysis of relapse was conducted using Cox proportional hazards models as per Swan et al30 on the 52 participants who had quit by 8 months. Small sample sizes precluded the ability to conduct exhaustive analyses.
RESULTS
The response rate at the 8-month PFH follow-up was 77% (n = 590), and 74% at the CCSS LT follow-up (n = 566). There were no differences in follow-up rate (mean = 74%) or time to the LT follow-up (mean = 38 months.) between conditions. There were no significant differences in quit rates between peer counselors for either the 8- or 12-month outcomes.
Demographics
Table 1 presents demographic characteristics for the original PFH cohort and the LT follow-up respondents. There were no significant differences among respondents and nonrespondents to the LT follow-up.
Table 1.
Demographics and Medical History
| Variable | Enrolled in PFH (n = 796)
|
Completed Long-Term Follow-Up (n = 565)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Self-Help
|
Peer Counseling
|
Self-Help
|
Peer Counseling
|
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Mean age, years | 31 | 31 | 31 | 31 | ||||
| SD | 6.8 | 6.5 | 6.9 | 6.5 | ||||
| Sex* | 216 | 54 | 209 | 52 | 147 | 52 | 141 | 50 |
| Marital status | ||||||||
| Married/cohabiting | 183 | 46 | 172 | 43 | 146 | 52 | 145 | 52 |
| Widowed/separated/divorced | 60 | 15 | 56 | 14 | 52 | 19 | 50 | 18 |
| Never married | 155 | 39 | 170 | 43 | 82 | 29 | 84 | 30 |
| Education | ||||||||
| < high school | 52 | 13 | 48 | 12 | 20 | 7 | 22 | 8 |
| High school/GED | 143 | 36 | 121 | 30 | 58 | 21 | 57 | 20 |
| Post-high school | 151 | 38 | 169 | 42 | 134 | 48 | 133 | 47 |
| College+ | 52 | 13 | 60 | 15 | 68 | 24 | 69 | 25 |
| Employed | 319 | 80 | 317 | 80 | 227 | 80 | 225 | 80 |
| Median cigarettes smoked per day at PFH baseline | 12 | 12 | 12 | 12 | ||||
| Range | 1-100 | 1-100 | 1-100 | 1-100 | ||||
NOTE. Enrolled values assessed at PFH baseline, long-term values assessed at long-term follow-up survey.
Abbreviations: PFH, Partnership for Health; SD, standard deviation; GED, general equivalency degree.
Percent male.
Smoking Cessation Outcomes
Using intention to treat analyses, 19% of all participants reporting having quit smoking at the LT follow-up. Quit rates at LT follow-up were significantly higher in the PC condition compared to SH (20.6% v 17.6%; P < .0003). This reflects a higher prevalence of cessation than reported at the 8-month follow-up, as it includes both continued cessation among those quit at the 8-month follow-up and subsequent cessation among those still smoking at 8 months.
Looking at the four-category outcome variable that considers smoking status at both 8-month and LT-follow-up points, in bivariate analyses SH participants were almost twice as likely to be continuous smokers at the LT follow-up versus continuous quitters, compared with those in the PC condition, although the difference did not reach statistical significance (odds ratio, 1.86; 95% CI, 0.975 to 3.55; P = .0591). Although smoking cessation rates continued to be higher among the PC group than SH, relapse rates were also higher in PC (11% v 4%), but not significantly different when compared with continuous quitters. There were no differences between conditions in the rate of quitting by the LT follow-up among those who reported being smokers at the 8-month follow-up (approximately 13% in both conditions). Overall, intervention condition was significantly associated with LT outcomes in bivariate analyses (P < .01).
Predictors of Smoking Outcomes at the Long-Term Follow-Up
There were significant relationships between smoking status at LT follow-up and nicotine dependence, use of nicotine replacement therapy (NRT), educational level, short-term and situational self-efficacy, and readiness to change (Table 2). To ensure against type I error, we would use caution in concluding statistical significance at a P = .003 level or higher. In multivariate analyses, use of NRT, education, sex, and situational self-efficacy, smoking rate, and stage of change remained significant; there was a trend toward significance for intervention condition (P = .0842; Table 3). Tests of potential mediation effect on intervention group determined that the only variable that mediated the outcome was use of nicotine replacement. Although nicotine replacement was available to all participants, it was used at a higher level by the PC group.
Table 2.
Bivariate Polytomous Logistic Regression Models Predicting Long-Term Smoking Status
| Independent Variable | Continuous Smoker v Continuous Quitter
|
Relapsed at Follow-Up v Continuous Quitter
|
Quit at Follow-Up v Continuous Quitter
|
Overall P for Significance | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Intervention condition | |||||||
| SH | 1.86 | 0.98 to 3.55 | 0.64 | 0.27 to 1.48 | 1.59 | 0.77 to 3.32 | .0018 |
| Peer counseling | 1.0 | 1.0 | 1.0 | ||||
| Sex | |||||||
| Female | 0.66 | 0.35 to 1.24 | 0.54 | 0.24 to 1.22 | 0.63 | 0.31 to 1.30 | .5068 |
| Male | 1.0 | 1.0 | 1.0 | ||||
| Age, 1-year increment from mean of 30.8 years* | 0.94 | 0.90 to 0.98 | 0.92 | 0.87 to 0.98 | 0.95 | 0.90 to 1.00 | .0363 |
| Nicotine dependence | |||||||
| Yes | 2.08 | 1.09 to 3.99 | 1.18 | 0.52 to 2.67 | 1.53 | 0.74 to 3.19 | .0251 |
| No | 1.0 | 1.0 | 1.0 | ||||
| Race/ethnicity | |||||||
| Nonwhite | 1.81 | 0.54 to 6.03 | 0.23 | 0.02 to 2.32 | 1.68 | 0.45 to 6.29 | .1772 |
| White | 1.0 | 1.0 | 1.0 | ||||
| Education, at follow-up | |||||||
| < high school | 2.96 | 0.63 to 13.85 | 1.22 | 0.18 to 8.46 | 0.42 | 0.05 to 3.32 | .0169 |
| High school | 1.98 | 0.75 to 5.23 | 0.70 | 0.19 to 2.60 | 1.20 | 0.41 to 3.53 | |
| Posthigh school | 1.66 | 0.78 to 3.54 | 0.38 | 0.13 to 1.14 | 1.15 | 0.50 to 2.65 | |
| College | 1.0 | ||||||
| Marital status, at follow-up | |||||||
| Single | 2.85 | 1.21 to 6.75 | 0.80 | 0.22 to 2.85 | 1.80 | 0.70 to 4.64 | .0077 |
| Widow/divorce | 3.03 | 1.02 to 9.95 | 1.12 | 0.25 to 4.97 | 1.98 | 0.61 to 6.46 | |
| Married | 1.0 | ||||||
| Cancer type | |||||||
| Bone/STS/Wilms’ | 0.75 | 0.36 to 1.55 | 1.04 | 0.42 to 2.60 | 0.55 | 0.24 to 1.29 | .6928 |
| CNS | 0.70 | 0.31 to 1.59 | 0.79 | 0.27 to 2.31 | 0.62 | 0.24 to 1.61 | |
| Leukemia/lymphoma | 1.0 | ||||||
| Used nicotine replacement therapy | |||||||
| No | 2.71 | 1.33 to 5.52 | 0.57 | 0.25 to 1.33 | 1.29 | 0.57 to 2.89 | .0001 |
| Yes | 1.0 | 1.0 | 1.0 | ||||
| Cancer at follow-up | |||||||
| Yes | 1.15 | 0.56 to 2.32 | 1.25 | 0.52 to 2.98 | 1.36 | 0.62 to 2.97 | .7907 |
| No | 1.0 | 1.0 | 1.0 | ||||
| Household income | |||||||
| < 19,999 | 4.71 | 1.07 to 20.77 | 1.50 | 0.16 to 14.50 | 2.50 | 0.41 to 15.29 | .1673 |
| 20,000-39,999 | 1.75 | 0.51 to 5.96 | 0.71 | 0.10 to 5.20 | 1.93 | 0.42 to 8.93 | |
| 40,000-59,999 | 2.29 | 0.62 to 8.53 | 2.50 | 0.36 to 17.39 | 2.88 | 0.58 to 14.33 | |
| 60,000-79,999 | 1.26 | 0.34 to 4.77 | 1.50 | 0.20 to 11.14 | 2.00 | 0.39 to 10.20 | |
| 80,000-99,999 | 2.59 | 0.42 to 15.90 | 4.00 | 0.36 to 44.35 | 4.00 | 0.50 to 32.13 | |
| More than 100,000 | Referent | ||||||
| No. of cigarettes/day, 1 cigarette increment from mean of 14.4* | 1.07 | 1.03 to 1.12 | 1.04 | 0.98 to 1.09 | 1.04 | 0.99 to 1.09 | .0002 |
| Age at first smoking, 1 year increment from mean of 17 years* | 0.98 | 0.90 to 1.07 | 1.03 | 0.90 to 1.16 | 1.02 | 0.92 to 1.12 | .5790 |
| Situational self-efficacy, 1 unit increment from mean of 8.59* | 0.87 | 0.78 to 0.98 | 0.91 | 0.79 to 1.05 | 0.87 | 0.76 to 0.98 | .0973 |
| Confident can quit smoking in 1 month | |||||||
| Not at all | 7.60 | 2.76 to 20.92 | 2.00 | 0.46 to 8.65 | 5.38 | 1.46 to 19.88 | .0003 |
| A little | 5.50 | 1.86 to 16.23 | 5.25 | 1.23 to 22.44 | 4.00 | 1.00 to 16.03 | |
| Somewhat | 5.27 | 1.88 to 14.83 | 2.80 | 0.66 to 11.95 | 4.00 | 1.05 to 15.26 | |
| Very | 3.27 | 0.96 to 11.20 | 2.80 | 0.53 to 14.78 | 5.76 | 1.29 to 25.71 | |
| Extremely | 1.0 | ||||||
| Short-term self-efficacy | |||||||
| Low | 3.58 | 1.69 to 7.62 | 1.99 | 0.74 to 5.33 | 1.70 | 0.72 to 4.00 | .0098 |
| Medium | 2.81 | 1.17 to 6.75 | 1.66 | 0.53 to 5.19 | 1.41 | 0.52 to 3.85 | |
| High | 1.0 | ||||||
| Situational self efficacy, 1 unit change from mean of 8.59* | 0.87 | 0.78 to 0.98 | 0.91 | 0.79 to 1.05 | 0.87 | 0.76 to 0.98 | .0973 |
| Stage of change at baseline | |||||||
| Precontemplation | 1.16 | 0.51 to 2.66 | 0.38 | 0.12 to 1.25 | 0.54 | 0.20 to 1.46 | .0200 |
| Early contemplation | 1.65 | 0.73 to 3.76 | 0.51 | 0.17 to 1.55 | 1.16 | 0.46 to 2.90 | |
| Contemplation | 1.86 | 0.67 to 5.14 | 1.04 | 0.30 to 3.55 | 0.97 | 0.30 to 3.09 | |
| Preparation | 1.0 | ||||||
NOTE. Reference group being continuous quitters to 8 months to long-term follow-up.
Abbreviations: OR, odds ratio; SH, self-help; STS, soft tissue sarcoma.
These variables are coded as continuous variables in the model, the referent value is the mean.
Table 3.
Multivariable Model for Long-Term Smoking Cessation Outcome*
| Independent Variable | Continuous Smoker v Continuous Smoke Free
|
Relapsed at Follow-Up vContinuous Smoke Free
|
Quit at Follow-Up v Continuous Smoke Free
|
Overall P for Significance | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Intervention condition | |||||||
| Self-help | 1.79 | 0.77 to 4.16 | 0.54 | 0.15 to 2.00 | 2.05 | 0.81 to 5.25 | .0842 |
| Peer counseling | 1.0 | 1.0 | 1.0 | ||||
| Sex | |||||||
| Female | 0.81 | 0.32 to 2.05 | 0.28 | 0.08 to 1.02 | 0.38 | 0.14 to 1.06 | .0420 |
| Male | 1.0 | 1.0 | 1.0 | ||||
| Education, at follow-up | .0009 | ||||||
| < HS | 3.72 | 0.98 to 14.06 | 0.86 | 0.15 to 5.12 | 0.55 | 0.12 to 2.49 | |
| Post HS | 2.45 | 0.84 to 7.14 | 0.29 | 0.06 to 1.31 | 0.90 | 0.29 to 2.84 | |
| College | 1.0 | ||||||
| Used nicotine replacement therapy | |||||||
| No | 3.68 | 1.26 to 10.75 | 0.94 | 0.23 to 3.88 | 1.07 | 0.34 to 3.39 | .0049 |
| Yes | 1.0 | 1.0 | 1.0 | ||||
| Situational self-efficacy, 1 unit increment from mean of 8.59* | 0.90 | 0.76 to 1.08 | 0.82 | 0.64 to 1.04 | 0.79 | 0.66 to 0.96 | .0574 |
| No. of cigarettes/day, 1 cigarette increment from mean of 14.4* | 1.05 | 0.98 to 1.12 | 0.96 | 0.88 to 1.06 | 1.01 | 0.94 to 1.08 | .0361 |
| Stage of change at baseline | |||||||
| Precontemplation | 0.59 | 0.15 to 2.30 | 0.13 | 0.02 to 1.11 | 0.23 | 0.05 to 1.10 | .0478 |
| Early contemplation | 1.57 | 0.45 to 5.45 | 0.21 | 0.03 to 1.45 | 0.64 | 0.16 to 2.54 | |
| Contemplation | 3.10 | 0.71 to 13.61 | 0.63 | 0.09 to 4.61 | 1.24 | 0.24 to 6.31 | |
| Preparation | 1.0 | ||||||
| Household income† | .9327 | ||||||
| Age at first smoking† | .7840 | ||||||
| Confident can quit smoking in 1 month† | .4984 | ||||||
| Age† | .6132 | ||||||
| Nicotine dependence† | .9652 | ||||||
| Race/ethnicity† | .9720 | ||||||
| Marital status, at follow-up† | .1436 | ||||||
| Cancer type† | .5948 | ||||||
| Cancer at follow-up† | .7933 | ||||||
| Length of time from baseline to long-term follow-up† | .2280 | ||||||
Abbreviations: OR, odds ratio; HS, high school.
Excluded short-term self-efficacy due to colinearity with situational self-efficacy.
These variables are nonsignificant in the model-only presenting P.
Quit Attempts Among Continued Smokers
Among those who were still smoking at the LT follow-up (n = 392), attempts to quit were not different by condition (at least 1 attempt: 58.7% of SH and 54% PC; 3+ attempts: 30.35% SH and 26.7% PC). Thus, the intervention did not have an impact on efforts to quit, but rather on the likelihood that those efforts would be successful.
Predictors of quit attempts.
Bivariate analyses explored relationships between quit attempts and potential mediating/moderating variables (Table 4). Having had radiation treatment was associated with quit attempts (P < .05), as was number of cigarettes smoked (heavier smokers less likely to make attempts; evaluated as a 1-unit change from mean of 15.2 cigarettes/day), self-efficacy, and readiness to change. In multivariate analyses, only stage of readiness to quit and number of cigarettes smoked predicted quit attempts at by the LT follow-up (Table 5).
Table 4.
Bivariate Polytomous Logistic Regression Models Predicting No. of Quit Attempts in Past 12 Months for Those Who Reported Smoking at Long-Term Follow-Up (N = 392)
| Independent Variable | No. of Attempts
|
Overall P for Significance | |||||
|---|---|---|---|---|---|---|---|
| None v 6 or More
|
1 or 2 v 6 or More
|
3, 4, or 5 v 6 or More
|
|||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Intervention condition | |||||||
| Self-help | 0.59 | 0.30 to 1.16 | 0.70 | 0.34 to 1.44 | 0.63 | 0.29 to 1.36 | .4688 |
| Peer counseling | 1.0 | ||||||
| Sex | |||||||
| Female | 1.71 | 0.86 to 3.39 | 1.88 | 0.91 to 3.88 | 1.75 | 0.80 to 3.81 | .3715 |
| Male | 1.0 | ||||||
| Age, 1-year increment from mean of 30.5 years* | 1.03 | 0.98 to 1.08 | 1.00 | 0.95 to 1.05 | 1.02 | 0.96 to 1.08 | .4849 |
| Nicotine dependence | |||||||
| Yes | 2.16 | 1.10 to 4.24 | 1.95 | 0.95 to 3.97 | 1.53 | 0.71 to 3.30 | .1315 |
| No | 1.0 | ||||||
| Race/ethnicity | |||||||
| Nonwhite | 0.93 | 0.35 to 2.46 | 0.31 | 0.09 to 1.07 | 0.84 | 0.27 to 2.63 | .1669 |
| White | 1.0 | ||||||
| Education at follow-up | |||||||
| ≤ HS | 1.54 | 0.56 to 4.25 | 1.36 | 0.46 to 4.01 | 1.53 | 0.49 to 4.76 | .6334 |
| Post HS | 0.72 | 0.31 to 1.70 | 0.85 | 0.34 to 2.11 | 0.67 | 0.25 to 1.79 | |
| College | 1.0 | ||||||
| Marital status at follow-up | |||||||
| Single | 0.85 | 0.41 to 1.75 | 1.26 | 0.59 to 2.71 | 1.02 | 0.44 to 2.37 | .2727 |
| Widow/divorce | 4.02 | 1.14 to 14.14 | 3.72 | 1.01 to 13.77 | 3.65 | 0.94 to 14.16 | |
| Married | 1.0 | ||||||
| Cancer type | |||||||
| Bone/STS/Wilms’ | 0.89 | 0.40 to 1.99 | 1.34 | 0.58 to 3.10 | 1.14 | 0.46 to 2.83 | .5618 |
| CNS | 0.54 | 0.22 to 1.28 | 0.66 | 0.26 to 1.67 | 0.85 | 0.32 to 2.28 | |
| Leukemia/lymphoma | 1.0 | ||||||
| Treatment type, radiation | |||||||
| Yes | 1.03 | 0.53 to 2.00 | 1.60 | 0.78 to 3.28 | 0.66 | 0.31 to 1.41 | .0459 |
| No | |||||||
| Use of nicotine replacement therapy | |||||||
| No | 0.70 | 0.22 to 2.21 | 0.54 | 0.16 to 1.77 | 0.39 | 0.12 to 1.34 | .3758 |
| Yes | 1.0 | ||||||
| Cancer at follow-up | |||||||
| Yes | 0.73 | 0.36 to 1.45 | 0.73 | 0.35 to 1.52 | 0.71 | 0.32 to 1.58 | .8109 |
| No | 1.0 | ||||||
| No. of cigarettes/day from mean of 14* | 1.08 | 1.03 to 1.12 | 1.07 | 1.02 to 1.12 | 1.06 | 1.01 to 1.11 | .0153 |
| Confident can quit smoking in 1 month | |||||||
| Not at all/a little | 3.85 | 1.52 to 9.75 | 2.44 | 0.93 to 6.40 | 5.26 | 1.46 to 19.04 | .0261 |
| Somewhat | 1.62 | 0.60 to 4.38 | 1.38 | 0.50 to 3.85 | 4.00 | 1.06 to 15.14 | |
| Very/extremely | 1.0 | ||||||
| Confident can quit smoking in 6 months | |||||||
| Not at all/a little | 3.97 | 1.65 to 9.52 | 1.90 | 0.75 to 4.82 | 1.22 | 0.45 to 3.27 | .0022 |
| Somewhat | 1.71 | 0.78 to 3.76 | 1.64 | 0.73 to 3.67 | 0.86 | 0.36 to 2.07 | |
| Very/extremely | 1.0 | ||||||
| Short-term self-efficacy | |||||||
| Low | 3.85 | 1.52 to 9.75 | 2.44 | 0.93 to 6.40 | 5.26 | 1.46 to 19.04 | .0261 |
| Medium | 1.62 | 0.60 to 4.38 | 1.38 | 0.50 to 3.86 | 4.00 | 1.06 to 15.14 | |
| High | 1.0 | ||||||
| Stage of change at baseline | |||||||
| Precontemplation | 7.36 | 2.31 to 23.43 | 1.31 | 0.37 to 4.65 | 0.75 | 0.19 to 2.98 | < .0001 |
| Early contemplation | 2.14 | 0.94 to 4.86 | 1.24 | 0.54 to 2.87 | 0.42 | 0.16 to 1.12 | |
| Contemplation | 2.46 | 0.92 to 6.61 | 1.36 | 0.49 to 3.76 | 0.79 | 0.26 to 2.34 | |
| Preparation | 1.0 | ||||||
NOTE. Referent group being 6 or more attempts.
Abbreviations: OR, odds ratio; HS, high school; STS, soft tissue sarcoma.
These variables are coded as continuous variables in the model, the referent value is the mean.
Table 5.
Multivariable Model for Quit Attempts Outcome at Long-Term Follow-Up
| Independent Variable | Continuous Smoker v Continuous Quitter
|
Relapsed at Follow-Up v Continuous Quitter
|
Quit at Follow-Up v Continuous Quitter
|
Overall P for Significance | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Intervention condition | |||||||
| Self-help | 0.60 | 0.29 to 1.22 | 0.69 | 0.33 to 1.43 | 0.67 | 0.31 to 1.48 | .5644 |
| Peer counseling | 1.0 | ||||||
| No. of cigarettes smoked/day from mean of 14 | 1.08 | 1.03 to 1.13 | 1.08 | 1.03 to 1.12 | 1.06 | 1.01 to 1.12 | .0234 |
| Stage of change at baseline | |||||||
| Precontemplation | 7.36 | 2.28 to 23.77 | 1.32 | 0.37 to 4.72 | 0.76 | 0.19 to 3.03 | < .0001 |
| Early contemplation | 2.11 | 0.91 to 4.93 | 1.21 | 0.52 to 2.86 | 0.42 | 0.16 to 1.11 | |
| Contemplation | 1.91 | 0.69 to 5.31 | 1.06 | 0.37 to 3.02 | 0.63 | 0.20 to 1.93 | |
| Preparation | 1.0 | ||||||
Abbreviation: OR, odds ratio.
Impact of intervention dose.
There were a maximum of six counseling calls (range, zero to six). Continuous quit rates were significantly higher in those who received five to six calls (11%) versus those who received 3 to 4 calls (5%) or 0 to 2 calls (3%; P < .0001). However, relapse rates among those who quit at 8 months were also higher among those with a higher intervention dose (17%, 11%, and 2%, respectively).
Subanalyses by length of follow-up.
Exploratory analyses examined the impact of length of follow-up on smoking status among those with follow-up duration of 3 to 4 years (n = 408 using intention to treat), and 4 or more years (n = 194). For 3 to 4 years of follow-up, there were significantly more continuous smokers in SH (71% v 60%). There were also fewer patients who relapsed in SH (3.3% v 10.5%). There were also small differences between groups in terms of being a delayed quitter at follow-up and a continuous quitter (SH v PC, 18% v 20%; 7.3% v 9.9%, respectively). The final multivariate model predicting LT smoking status in those with 3 to 4 years of follow-up revealed nicotine replacement (NRT) use (P = .0013) and education (P ≤ .0001) as significant predictors, and a trend toward significance in intervention condition (P = .0552).
Similar bivariate patterns were found in the group with 4+ years of follow-up, although there were no continuous quitters in the SH group (SH v PC, 0% v 6.7%). For 4+ years of follow-up, in the final model only NRT use was significant (P < .002). Caution should be used in interpreting these results due to the small sample size.
Subanalysis of relapse.
A Cox proportional hazards model was used to analyze relapse rates for the 52 participants who had quit smoking at the 8-month follow-up; only age (higher for younger participants) and depression at the 8-month time point were significantly related to relapse. There was a significant relationship between age and depression (older participants were less depressed), and thus only depression remained significantly related to relapse rates when both variables were entered into the model.
DISCUSSION
This article assesses the long-term impact of PFH, the first large-scale intervention targeting smoking cessation among childhood cancer survivors. Although the length of follow-up varies, this study provides an important opportunity to examine the experiences of smokers several years out from a smoking cessation program. Cessation rates continue to be significantly higher among participants in the PC condition versus SH, although the differences are not large. There was a trend toward higher continuous smoking rates among those in SH, with these participants being almost twice as likely to be smoking at every follow-up, compared with those in PC; PC participants had more active engagement with smoking cessation throughout the follow-up period.
Long-term cessation rates were lower among men, those who were nicotine dependent, and those of lower educational levels, and were higher among those who used NRT and who had higher self-efficacy. These mediators are similar to those typically found in the literature. In particular, it is important to note that use of NRT, which was provided at no cost, increased LT outcomes. Increasing NRT use among survivors would be consistent with Agency for Health Care Policy and Research guidelines.31 Further, recent work has also found that offering free NRT in the general population of smokers through a tobacco quit line increases both quit line utilization and quit rates as previously reported.32,33 Recent studies using ecological momentary assessment have elucidated the role of fluctuating self-efficacy in the onset of lapses.34 There is also some very recent work that is beginning to look at the role of self-efficacy versus satisfaction with outcomes in longer-term maintenance.35 Future work with survivors should explore the differential role of self-efficacy and satisfaction with outcomes as key predictors of cessation.
Relapse rates, although higher in the PC group, were not significantly different. It is unclear if additional support or continued counseling would have been helpful to these smokers, or if relapse was an effect of the withdrawal of support. The fact that both continued cessation rates and relapse rates were higher among those receiving a higher intervention dose suggests that greater understanding of the characteristics of individuals who might benefit from a higher dose is needed. Some individuals may benefit from self-directed ongoing support, such as through a web site.
There are few studies that address long-term quit rates in randomized control trials. Kadowaki et al36 reported 5-year cessation rates of 10.9% among 251 Japanese men in a physician-delivered smoking intervention. Cessation rates at 2.5 years among Veterans’ Affairs patients were 17.6% to 20.3%, with no between-group differences.37 Manfredi et al38 reported quit rates of 11% (no group differences) at 18 months in public health smoking clinics for women. Overall, there are few examples in the literature of interventions that have yielded long-term intervention effects.
In 2000, Ockene and colleagues39 reviewed the evidence on LT maintenance for smoking cessation and recommended that the field systematically move to longer follow-up assessments, with a minimum of 2-year follow-ups. There has only been limited movement in this direction. The recent Cochrane review of telephone counseling interventions for smoking cessation reported that the longest follow-up for any studies reviewed was 12 months;40 rarely do follow-up periods extend beyond 1 year.41 This study took advantage of an opportunity to collect LT outcomes, but the follow-up period varied (see limitations below). Exploratory subgroup analyses by similar follow-up periods confirmed the overall findings of higher rates of continuous smoking in SH, and higher rates of both quitting and relapse in the PC condition. Of note, in the analysis of those with 4+ years of follow-up data, there were no continuous quitters in the SH group, versus almost 7% in the PC group. Overall, there was a higher level of engagement with cessation among the PC group over the follow-up periods examined. This is important, as younger smokers are typically less likely to quit, and activating the cessation process as early as possible is critical given survivors’ health vulnerabilities.
This study highlights the need to develop an effective infrastructure for delivery of smoking cessation services to childhood cancer survivors. A recent study of clinics treating childhood cancer survivors found that only 3% of programs follow national guidelines on treating smoking in the health-care setting.42 Further, this study demonstrated that the infrastructure for identifying survivors within treatment/LT care programs is largely missing, and a more systematic approach to patient tracking and follow-up is needed.
LT smoking outcomes and quit attempts were not associated with subsequent cancer diagnosis, although recurrence was common (32% reported a cancer or benign tumor at follow-up). Recurrence or new diagnoses may offer a teachable moment for those who continue to smoke, although it would be important not to place blame for recurrence on continued smoking.43,44
Study limitations should be noted. First, the response rate to PFH was impacted by difficulties in reaching potential participants in this young and highly mobile population, although retention among those enrolled was high (> 70%), particularly given the length of the follow-up period. Intention-to-treat analyses assessed long-term outcomes, conservatively assuming that those who did not respond were smokers. Another limitation was the variable length of the long-term follow-up interval. Although it would be have been ideal to have had a standardized length of follow-up, accrual to CCSS was done on a rolling basis, which meant differing lengths of time between the PFH and CCSS follow-ups. Although not ideal, the follow-up approach utilized represented efforts to be cost-efficient, and to take advantage of long-term CCSS follow-up to assess smoking outcomes in PFH participants. It is extremely difficult to maintain an intervention cohort over several years after intervention completion, and thus there are few studies in the literature that evaluate LT outcomes. Thus, the added benefit of this unique data may off-set this methodologic limitation to some extent. Use of self-report cessation outcomes is also a limitation in population-level studies such as PFH.45 However, the PFH outcomes evaluation did use the bogus pipeline procedure, a well-accepted strategy for increasing the accuracy of self-report.46
There are several important strengths to note. The study sample was drawn from the largest and most comprehensively characterized research cohort of childhood cancer survivors.17 The large sample of smokers provided sufficient power to detect study outcomes. Data were conservatively analyzed using intention to treat. This study also highlights the importance of cohort studies in general, and the CCSS Long-Term Follow-Up Study of cancer survivors in particular. There are significant barriers to conducting long-term follow-up of behavior change interventions. This study of LT outcomes in PFH was possible only because we were able to embed it in the context of the CCSS data collection effort, which was separately funded.
It is particularly important to activate smoking cessation efforts among childhood cancer survivors as early as possible in order to decrease the chances of the deleterious health effects of smoking. These findings suggest that efforts should be made to increase smoking cessation among survivors, and that providing brief interventions lead to improved LT outcomes, which may improve health outcomes of childhood cancer survivors overall. The impact of the PFH intervention was significant but relatively small, although not atypical of smoking cessation programs that target all smokers, including those who are not interested in quitting. Further, it is important to consider the population-level impact of such findings, which can be considerable in shifting distribution of disease.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Karen M. Emmons, Elaine Puleo, Ann Mertens, Ellen R. Gritz, Lisa Diller, Frederick P. Li
Financial support: Karen M. Emmons, Ann Mertens, Frederick P. Li
Provision of study materials or patients: Karen M. Emmons
Collection and assembly of data: Karen M. Emmons, Ann Mertens, Lisa Diller
Data analysis and interpretation: Karen M. Emmons, Elaine Puleo, Ellen R. Gritz, Lisa Diller
Manuscript writing: Karen M. Emmons, Elaine Puleo, Ann Mertens, Ellen R. Gritz, Lisa Diller, Frederick P. Li
Final approval of manuscript: Karen M. Emmons, Elaine Puleo, Ann Mertens, Ellen R. Gritz, Lisa Diller, Frederick P. Li
published online ahead of print at www.jco.org on December 1, 2008
Supported by Grants no. U24-CA55727, RO1-CA77780, and R01CA106914-04 from the National Institutes of Health; support provided to the University of Minnesota by the Children's Cancer Research Fund; and support provided to the Dana-Farber Cancer Institute by Liberty Mutual, the Patterson Fellowship Fund, and the Harry and Elsa Jiler American Cancer Society Research Professorship (F.P.L.). Smith Kline Beecham donated nicotine patches.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Haupt R, Fears T, Robison L, et al: Educational attainment in long-term survivors of childhood acute lymphoblastic leukemia. JAMA 272:1427-1432, 1994 [PubMed] [Google Scholar]
- 2.Ries LAG, Percy CL, Bunin GR: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, MD, Cancer Statistics Branch, National Cancer Institute, 1999, pp 1-15
- 3.National Cancer Policy Board: Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington DC, National Academy Press, 2003 [PubMed]
- 4.Meadows A: Curing cancer in children: Minimizing price, maximizing value. J Clin Oncol 13:1837-1839, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Robison L, Oberlin O, et al: Breast cancer and other second neoplasms after childhood Hodgkin's disease. NE J Med 334:745-751, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Bouland F, Sands S, Sklar C: Late complications after bone marrow transplantation in children and adolescents. Curr Probl Pediatr 28:1303-1329, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Robison LL, Mertens A: Second tumors after treatment of childhood malignancies. Hematol Oncol Clin North Am 7:401-415, 1993 [PubMed] [Google Scholar]
- 8.Marina N: Long-term survivors of childhood cancer: The medical consequences of cure. Pediatr Clin North Am 44:1021-1042, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz CL: Late effects of treatment in long-term survivors of cancer. Cancer Treatment Review 21:355-366, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Gurney JG, Kadan-Lottick NS, Packer RJ, et al: Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor study. Cancer 97:663-673, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Trichopoulos D, Li FP, Hunter DJ: What causes cancer? Sci Am 275:80-87, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Leung W, Hudson MM, Strickland DK, et al: Late effects of treatment in survivors of childhood acute myeloid leukemia. J Clin Oncol 18:3273-3279, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Tao ML, Guo MD, Weiss R, et al: Smoking in adult survivors of childhood acute lymphoblastic leukemia. J Natl Cancer Inst 90:219-225, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Larcombe I, Mott M, Hunt L: Lifestyle behaviours of young adult survivors of childhood cancer. Br J Cancer 87:1204-1209, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stull V, Snyder D, Demark-Wahnefried W: Lifetsyle interventions in cancer survivors: Designing programs that meet the needs of this vulnerable and growing population. J Nutr 137:243S-248S, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Emmons K, Li FP, Whitton J, et al: Predictors of smoking initiation and cessation among childhood cancer survivors: A report from the childhood cancer survivor study. J Clin Oncol 20:1608-1616, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Robison L, Mertens A, Boice J, et al: Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol 38:229-239, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Mertens A, Potter J, Neglia J, et al: Methods for tracing, contacting, and recruiting a cohort of survivors of childhood cancer. J Pediatr Hematol Oncol 19:212-219, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Emmons K, Butterfield R, Puleo E, et al: Smoking among participants in the Childhood Cancer Survivors Cohort: The Partnership for Health Study. J Clin Oncol 21:189-196, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Emmons K, Puleo E, Park E, et al: Peer-delivered smoking counseling for childhood cancer survivors increases smoking cessation: Outcomes of the Partnership for Health Study. J Clin Oncol 23:6516-6523, 2005 [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute: Clearing the Air: How to Quit Smoking and Quit for Keeps. Washington DC, US Department of Health and Human Services, Public Health Service, National Institutes of Health, 1993
- 22.Fagerstrom KO, Schneider NG: Measuring nicotine dependence: A review of the Fagerstrom Tolerance Questionnaire. J Behav Med 12:159-182, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Gritz E, Kristeller J, Burns D: Treating nicotine addiction in high risk groups and patients with medical co-morbidity, in Orleans C, Lode J (eds): Nicotine Addiction: Principles and Management. New York, NY, Oxford University Press, 1993
- 24.Prochaska JO, DiClemente CC, Norcross JC: In search of how people change: Applications to addictive behaviors. Am Psychol 47:1102-1114, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Andresen EM, Malmgren JA, Carter WB, et al: Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 10:77-84, 1994 [PubMed] [Google Scholar]
- 26.Whooley MA, Avins AL, Miranda J, et al: Case-finding instruments for depression: Two questions are as good as many. J Gen Intern Med 12:439-445, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware JE, Sherbourne CD: The MOS 36-item short form health survey (SF-36): Conceptual framework and item selection. Med Care 30:473-483, 1992 [PubMed] [Google Scholar]
- 28.Jorenby DE, Leischow SJ, Nides MA, et al: A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 340:685-691, 1999 [DOI] [PubMed] [Google Scholar]
- 29.MacKinnon DP, Dwyer JH: Estimating mediated effects in prevention studies. Evaluation Review 17:144-158, 1993 [Google Scholar]
- 30.Swan GE, Ward MM, Jack LM: Abstinence effects as predictors of 28-day relapse in smokers. Addict Behav 21:481-490, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Agency for Healthcare Research and Quality: Treating Tobacco Use and Dependence: A Systems Approach.: A Guide for Health Care Administrators, Insurers, Managed Care Organizations, and Purchasers. 2000. http://www.ahrq.gov/clinic/tobacco/systems.htm
- 32.Tinkelman D, Wilson SM, Willett J, et al: Offering free NRT through a tobacco quitline: Impact on utilisation and quit rates. Tob Control 16:i42–i46, 2007. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandura A: Self-Efficacy: The Exercise of Control. New York, NY, W.H. Freeman and Company, 1997
- 34.Gwaltney CJ, Shiffman S, Balabanis MH, et al: Dynamic self-efficacy and outcome expectancies: Prediction of smoking lapse and relapse. J Abnorm Psychol 114:661-675, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Baldwin AS, Rothman AJ, Hertel AW, et al: Specifying the determinants of the initiation and maintenance of behavior change: An examination of self-efficacy, satisfaction, and smoking cessation. Health Psychol 25:626-634, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Kadowaki T, Watanabe M, Okayama A, et al: Continuation of smoking cessation and following weight change after intervention in a healthy population with high smoking prevalence. J Occup Health 48:402-406, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Velicer WF, Friedman RH, Fava JL, et al: Evaluating nicotine replacement therapy and stage-based therapies in a population-based effectiveness trial. J Consult Clin Psychol 74:1162-1172, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Manfredi C, Crittenden KS, Cho YI, et al: Long-term effects (up to 18 months) of a smoking cessation program among women smokers in public health clinics. Prev Med 38:10-19, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Ockene JK, Emmons KM, Mermelstein RJ, et al: Relapse and maintenance issues for smoking cessation. Health Psychol 19:17-31, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Stead LF, Perera R, Lancaster T: Telephone counseling for smoking cessation. Cochrane Database Syst Rev 3:CD002850, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Klesges RC, DeBon M, Vander Weg MW, et al: Efficacy of a tailored tobacco control program on long-term use in a population of U.S. military troops. J Consult Clin Psychol 74:295-306, 2006 [DOI] [PubMed] [Google Scholar]
- 42.de Moor J, Puleo E, Butterfield R, et al: The availability of smoking prevention and cessation services for childhood cancer survivors. Cancer Causes Control 18:423-430, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Gritz ER, Fingeret MC, Vidrine DJ, et al: Successes and failures of the teachable moment: Smoking cessation in cancer patients. Cancer 106:17-27, 2006 [DOI] [PubMed] [Google Scholar]
- 44.McBride CM, Emmons KM, Lipkus IM: Understanding the potential of teachable moments: The case of smoking cessation. Health Educ. Res 18:156-170, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Velicer W, Prochaska J, Rossi J, et al: Assessing outcome in smoking cessation studies. Psychology Bulletin 111:23-41, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Hansen W, Malotte C, Fielding J: The bogus pipeline revisited: The use of the threat of detection as a means of increasing self-reports of tobacco use. J Appl Psychol 70:789-792, 1985 [PubMed] [Google Scholar]