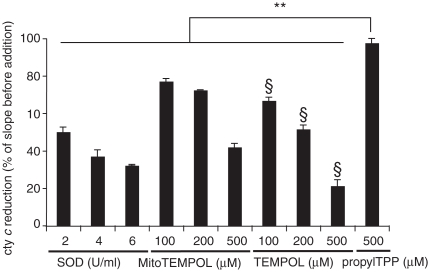
Figure 2.
Superoxide dismutase activity of MitoTEMPOL. Superoxide was produced by mixing 20 mU xanthine oxidase with 10 mM acetaldehyde in 50 mM potassium phosphate buffer (pH 8) supplemented with 500 μg/ml ferricytochrome c at 37°C. The rate of cytochrome c reduction was followed at 550 nm for 60 s after which the appropriate amount of SOD, MitoTEMPOL, TEMPOL or propylTPP was added and the rate was followed for another 60 s. The slope of the cytochrome c reduction progress curve was measured after each addition and is expressed as a percentage of the slope before the addition. Results are means ± SD of three measurements. ** p < 0.01; § p < 0.05 with respect to the same concentration of MitoTEMPOL.