Abstract
It is not readily apparent how pulmonary function could be improved by resecting portions of the lung in patients with emphysema. In emphysema, elevation in residual volume relative to total lung capacity reduces forced expiratory volumes, increases inspiratory effort, and impairs inspiratory muscle mechanics. Lung volume reduction surgery (LVRS) better matches the size of the lungs to the size of the thorax containing them. This restores forced expiratory volumes and the mechanical advantage of the inspiratory muscles. In patients with heterogeneous emphysema, LVRS may also allow space occupied by cysts to be reclaimed by more normal lung. Newer, bronchoscopic methods for lung volume reduction seek to achieve similar ends by causing localized atelectasis, but may be hindered by the low collateral resistance of emphysematous lung. Understanding of the mechanisms of improved function after LVRS can help select patients more likely to benefit from this approach.
Keywords: lung mechanics, emphysema surgery, lung recoil, airflow limitation, respiratory muscles
PHYSIOLOGIC BASIS FOR IMPAIRMENT WITH EMPHYSEMA
Inflammatory changes associated with chronic obstructive pulmonary disease (COPD) initiate the changes in lung mechanical properties that characterize emphysema. Inflammation leads to destruction of elastin, which contributes to lung elasticity, and collagen, which provides tensile strength. Although recent interest has focused on the cellular and molecular mechanisms contributing to emphysema, the study of lung mechanical stresses, one of the earliest postulated causes of emphysema (1), has received less attention. Weakened lung parenchyma is more likely to fracture during respiratory stress, and coalescence of a few alveoli can increase stress on adjacent units in a way that favors formation of large, localized cysts similar to the pattern seen in patients with advanced emphysema (2–4).
Effects of Emphysema on Expiratory Flow
These sequelae of inflammation ultimately decrease lung elastic recoil and small airway caliber, contributing to characteristic airflow limitation. The three classic determinants of expiratory flow limitation are lung elastic recoil, the propensity for airways to close, and airway resistance (5). Loss of elastic recoil in emphysema decreases the upstream pressure that drives expiratory flow, thereby decreasing maximal flows at any lung volume. Loss of elastic recoil also increases the unstressed volume of the lung (the volume remaining when elastic recoil is zero). Loss of radial traction on airways from lung parenchyma contributes to airway closure at higher lung volumes (increased trapped volume) because a higher transmural pressure is required to maintain airway patency. In addition, airway caliber at any lung volume is decreased (6). Coexisting small airway inflammation and fibrosis further increase airway resistance (7, 8). These changes collectively reduce the rate of lung emptying.
The rate of lung emptying may be expressed as its time constant, the time necessary for approximately 63% of the lung to empty. The time constant is the product of lung compliance and resistance; in emphysema, it is prolonged by both increased compliance and increased airway resistance. On the expiratory flow–volume curve, this can be seen as a decreased slope, which is in units of 1/time. Greater heterogeneity of rates of emptying among lung units makes the slope concave upward, as more rapidly emptying units dominate in early expiration and slowly emptying units dominate in later expiration. The prolongation is also manifested by decreased FEV1/FVC, which is inversely proportional to the time constant.
Consideration of only FEV1/FVC, however, overlooks a second important factor in the decreased FEV1 in emphysema. Although decreased FEV1/FVC is a hallmark of obstruction, it is also axiomatic that FEV1 is the product of FEV1/FVC multiplied by FVC:
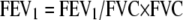 |
(1) |
This equation is simple to the point of tautology, but it focuses attention on an overlooked aspect of lung function that becomes central to understanding the effects of emphysema and of LVRS: FEV1 will be decreased by reductions in FVC. That is, FEV1 can be reduced if the lungs empty either more slowly or less completely. As will be shown, both factors are at play in emphysema.
Effects of Emphysema on Inspiratory Muscles
In addition to reducing maximal expiratory airflow, the parenchymal changes of emphysema have important effects on inspiratory effort. The altered balance between passive lung and chest wall recoils increases FRC. When tidal expiratory flow is sufficiently decreased, end-expiratory lung volume will exceed equilibrium FRC, especially when ventilation increases with exercise (dynamic hyperinflation).
Increased end-expiratory lung volume impairs the efficient operation of the inspiratory muscles. The diaphragm operates at a shorter resting length during tidal breathing, in an unfavorable position on its length–tension curve. This leads to decreased force of contraction for a given neural stimulus (9). Changes of in vivo diaphragm configuration also impair its effectiveness at lowering pleural pressure for a given tension. The muscle fibers are oriented more radially. The diaphragm is less able to descend during inspiration, and less effective at rotating the lower ribs outward.
Finally, increased end-expiratory lung volume also increases the load against which the inspiratory muscles must operate. Elastic recoil of the chest wall is directed inward at high volumes. Dynamic hyperinflation and premature airway closure means inspiratory effort begins while alveolar pressure is still positive, such that isovolumic inspiratory effort is expended to reduce alveolar pressure to ambient before inspiratory flow can commence. The curvilinear pressure–volume relationship of the lung requires a greater change in pleural pressure to distend the lung by a given tidal volume at higher lung volumes. Thus, dynamic hyperinflation reduces inspiratory muscle pressure-generating capacity while it increases pressure-generating demands, a situation predisposing to respiratory failure. Exertional dyspnea in COPD correlates with increased end-expiratory lung volume (10).
Coupling of the Lungs and the Inspiratory Muscles
The effects of emphysema on the lung and effects of hyperinflation on the inspiratory muscles are closely intertwined, and together reduce FEV1. Residual volume (RV) is increased due to premature airway closure and large volumes of air trapped in cysts and bullae. The relatively normal inspiratory muscles are incapable of expanding the emphysematous lung much above RV. Maximal elastic recoil pressure is low. Total lung capacity (TLC) increases, but not by as much as RV. Thus, the difference between these two volumes, VC, is reduced. Because FEV1 depends on, and can never exceed, FVC (Equation 1), FEV1 must fall. Any associated increase in time constants will also decrease FEV1. However, quantitative analysis has shown that decreased VC is the major factor in the reduction in FEV1 in both COPD and α1-antitrypsin deficiency (11). These changes are quite similar to what would be found if a pair of perfectly normal lungs from a very large person were transplanted into the chest of a small person.
PHYSIOLOGIC BASIS FOR IMPROVEMENT AFTER LUNG VOLUME REDUCTION SURGERY
As with any procedural therapy, some variability in outcomes is due to differences in operator skill that cannot be quantified. The purpose of this section is to understand the outcome variability that can be attributed to physiologic factors—that is, to measure that which can be measured, and perhaps supplement clinician judgment with quantifiable predictors of outcome. Otto Brantigan and colleagues' earliest reports of lung volume reduction surgery (LVRS) proposed that increased elastic recoil, increased radial traction on airways, and restoration of a more normal configuration of the respiratory muscles explained the clinical benefits (12). Fifty years later, these speculations remain roughly correct.
Increased Lung Elastic Recoil
Early physiologic studies of LVRS focused on increased elastic recoil and decreased airway resistance (13–17). For example, Gelb and coworkers showed that LVRS increased elastic recoil at a given lung volume (13, 14). It is intuitively obvious that removing a portion of lung would increase the recoil of the remaining lung that expands to fill the thorax (assuming that bullae do not expand instead). However, preoperative measurements of elastic recoil do not reliably predict response after LVRS, and improvement in recoil correlates poorly, if at all, with improvement in FEV1 (13, 16). In groups of patients undergoing LVRS, mean changes in FEV1/FVC (which would be expected to increase from an increase in recoil) are small or absent despite mean improvements in FEV1 (18, 19). Thus, although LVRS usually will increase recoil pressure at TLC, this does not fully explain increased FEV1.
Studies examining the effect of LVRS on airway resistance have also shown inconsistent findings. Gelb and coworkers (13) found LVRS increased the slope of the relationship between recoil pressure and maximum flow (greater flow at the same pressure), suggesting a decrease in airway resistance. In contrast, Scharf and colleagues (15) found no consistent changes in airway resistance using similar methods. Several of their subjects even showed marked increases in airway resistance. In these cases, airflow nevertheless rose, so the increased recoil was apparently sufficient to mask the increase in resistance. This heterogeneous response in airway caliber was attributed to distortion and/or kinking of the airways, as well as reduction of total airway cross-sectional area.
Increased VC
To better understand postoperative increases in FEV1, one must note that LVRS increases VC as consistently as it does FEV1. However, it is not immediately apparent how lung resection can allow a patient to exhale a larger volume of air.
Many studies have suggested that the best candidates for LVRS are those in whom emphysema is most heterogeneous, with localized target areas composed only of cysts and bullae (20–23). Consider the effects on RV and TLC after removal of such regions. Lung compliance would not change, because these empty spaces do not contribute to lung elasticity. RV would fall by the volume of the resected cysts and bullae. TLC would also fall. However, the inspiratory muscles can now stretch the remaining lung further. Therefore, the difference between TLC and RV, VC, increases (Figure 1A). From Equation 1, FEV1 increases because VC increases. Although lung recoil rises, this does not per se improve FEV1.
Figure 1.
(A) Effects of lung volume reduction surgery (LVRS) that removes only cysts and bullae. The dashed line represents the static relationship between pleural pressure and lung volume, as might be recorded as a subject with emphysema makes a very slow (quasi-static) inspiration from residual volume (RV) to total lung capacity (TLC). VC is represented by the difference on the ordinate between TLC and RV. Maximal elastic recoil pressure is shown by the double-headed arrows at TLC. The slope of the relationship is lung compliance. The line labeled “inspiratory muscle capacity” represents the chest wall pressure–volume relationship during maximal inspiratory muscle contraction. It could be recorded by measuring the maximal negative pleural pressure as a subject makes a series of inspiratory efforts against an occluded airway at various lung volumes. TLC is reached when the increasing recoil of the lung equals the diminishing maximal recoil of the chest wall. Effects of LVRS are shown by the thin vertical line. Because this LVRS removed only destroyed lung, which does not contribute to lung elastic properties, compliance is unchanged. RV is reduced, and TLC is reduced by a lesser amount because the muscles can stretch the remaining lung further. The difference between them, the VC, increases. Recoil pressure also increases, but this does not cause the increase in VC. (B) Effects of LVRS in a patient with diffuse emphysema. In this example, the resected lung includes parenchyma, which has some elastic recoil. Its removal decreases the compliance of the lung left behind. Note that now the recoil pressure rises by more than in (A), but the VC improves by less. If LVRS impairs intrinsic muscle function, the curve labeled “inspiratory muscle capacity” would shift downward. This would also limit the improvement in VC.
If emphysema is diffuse such that LVRS resects some portions of more normal lung as well as cysts, then lung compliance will fall, and maximal recoil pressure will increase further. However, the recoil of the less compliant lungs will hinder the ability of the inspiratory muscles to preserve TLC. The improvement in VC, and therefore in FEV1, will generally be less (Figure 1B). The baseline characteristics that predict the greatest improvement in FEV1 can be modeled mathematically (11). They are high lung compliance, low airway resistance, capable inspiratory muscles, and, most important, a high RV/TLC. This ratio expresses the mismatch between the size of the lungs and the size of the thorax. These characteristics, of course, are those of emphysema, and this model explains why LVRS would be useful in that disease but not in pulmonary fibrosis, despite increasing recoil much more in the latter.
A few studies have applied this model to patients undergoing LVRS. One used multivariate logistic regression to identify preoperative predictors of improvement in FVC and FEV1 in 83 patients who had undergone a variety of LVRS procedures (24). Equation 1 was applied to partition the improvement in FEV1 into its contributing factors. The data revealed both the strengths and weaknesses of the underlying mathematical model. As predicted by the model, the baseline RV/TLC was the only independent predictor of the change in FVC. When the subjects were divided into two groups based on RV/TLC above or below the median value (0.67), patients with low RV/TLC had no significant change in FVC, whereas those with high RV/TLC had improvement in FVC. Furthermore, 70% of the change in FEV1 could be attributed to the change in FVC.
However, the model was less successful at predicting the change in FEV1, which failed to correlate with the baseline RV/TLC. Subjects with low baseline RV/TLC had the same mean improvement in FEV1 (42 ± 12%) as those with high baseline RV/TLC (51 ± 6%). This indicates that changes in FEV1/FVC also contribute to the change in FEV1. Subjects with low baseline RV/TLC were more likely to have improved FEV1/FVC, and 86% of their improvement in FEV1 was attributable to increased FEV1/FVC. In contrast, among the high RV/TLC subjects, 79% of their FEV1 improvement was due to increased FVC. Thus, the theoretical model is limited in that it does not anticipate or explain the improvement in FEV1/FVC seen in some patients.
Some of these mechanisms were studied in greater detail in a group of patients who had lung and chest wall mechanics measured with esophageal balloon catheters before and after LVRS (25). The patients were categorized into responders and nonresponders on the basis of their improvement in FEV1 (an increase by 50% in the former and no significant change in the latter group). Differences between the groups were then explored. The responders had an increase in VC, whereas in nonresponders RV and TLC fell equally and VC was unchanged. Lung compliance fell and recoil pressure rose in both groups and, on average, neither group showed changes in the pressure at which airways closed or the airway resistance upstream from the site of flow limitation. Improvement in FEV1 was largely or wholly attributable to the increase in VC.
Many studies have documented that LVRS outcomes are better in patients with heterogeneous emphysema, especially of the upper lobes, than in those with diffuse emphysema (20, 22). Before the National Emphysema Treatment Trial (NETT), some centers selected only patients with heterogeneous emphysema for surgery (21). The NETT has confirmed better functional and symptomatic results in such patients (23). There are several potential explanations for this finding. First, as described above, resection limited to areas extensively replaced by cysts and bullae would come closest to removal of pure RV (Figure 1A). Second, large bullae may compress more normal lung. This can distort airways, cause microscopic areas of atelectasis, or impair the surface-active properties of the compressed lung. Similar findings have been described in normal subjects subjected to chest strapping (26). Allowing these compressed areas to inflate more fully could reverse these changes. This would attenuate the decrease in lung compliance after LVRS (maximizing improvement in VC), and could improve airway resistance. It is not known precisely which of these mechanisms explain the generally better outcomes among patients with heterogeneous, upper lobe emphysema.
In summary, improvement in FEV1 after LVRS is not due to increased lung recoil pressures or normalization of the elevated compliance of emphysema. LVRS better matches the size of the lungs to the capacity of the thorax which contains them. This increases VC, the major determinant of the increase in FEV1. In individual patients, improvement in the rate of lung emptying also contributes to increase FEV1.
Respiratory Muscle Function
Inspiratory muscle function may improve because of restoration of a normal mechanical advantage. However, the effects of LVRS may be more complicated than simply allowing tidal breathing to occur at a lower working volume.
LVRS increases the maximal force of contraction of the inspiratory muscles at RV or FRC, the expected result of decreased lung volumes (27, 28). This has also been shown specifically for the diaphragm, measured by maximal transdiaphragmatic pressure during sniffs, inspiratory/expulsive maneuvers, and phrenic stimulation (19). Cassart and colleagues used three-dimensional reconstruction of the diaphragm from computed tomography scans to show that LVRS increased the zone of apposition, which would improve the mechanical efficiency of inspiration (29). Increased zone of apposition has also been associated with decreased motor unit firing rates in the diaphragm and scalenes (30).
There are additional effects of LVRS that could impact respiratory muscle function. On one hand, decreased corticosteroid use and/or decreased PaCO2 may improve intrinsic muscle function. On the other hand, respiratory muscles may adapt to longstanding hyperinflation. With experimental emphysema, the diaphragm remodels to better match its length–tension relationship to its foreshortened resting length (31, 32). Similarly, patients with COPD may have better inspiratory muscle function than normal subjects at equal lung volumes (33). If LVRS countermands such adaptive mechanisms, then the expected improvements due to decreased lung volume alone may be attenuated. For example, in one study (25), inspiratory muscle function was measured with esophageal balloons. Before and after full recovery from LVRS, subjects made maximal inspiratory efforts against an occlusion at a range of lung volumes from RV to TLC. This yielded a pressure–volume relationship representing maximal muscle capacity (Figures 1A and 1B, the line labeled “inspiratory muscle capacity”). Surprisingly, LVRS depressed this relationship. That is, at any given lung volume, subjects could lower esophageal pressure less after surgery than before. This impairment of respiratory muscle function would undermine the spirometric benefits of surgery. The mechanism of this impairment is not known. Animal studies have shown diaphragmatic injury and dysfunction in the immediate postoperative period, attributed to acute stretching of the muscle (34). In patients, sniff inspiratory pressure improved between 6 weeks and 6 months after LVRS, suggesting inspiratory muscle recovery or remodeling (35).
In summary, LVRS improves inspiratory muscle function by reducing lung volume, thereby improving the mechanical advantage of the inspiratory muscles. However, intrinsic muscle function may be somewhat impaired after surgery, limiting the potential benefits.
PHYSIOLOGIC BASIS FOR IMPROVEMENT AFTER BRONCHOSCOPIC LUNG VOLUME REDUCTION
Several promising techniques for lung volume reduction (LVR) via a bronchoscope are under investigation (see Ingenito and coworkers, pages 454–460, this symposium [43]). Here, we compare the physiologic mechanisms of improvement of surgical versus endobronchial LVR.
Most bronchoscopic methods attempt to induce atelectasis. This may be achieved with one-way (expiratory) valves placed in the airways or by introduction of tissue glues (36–39). In patients in whom atelectasis is achieved, mechanisms of improved lung function or symptoms are likely identical to those after surgical LVR. Hopkinson and colleagues (37) compared patients who did or did not develop radiologic atelectasis after bronchoscopic valve placement. Atelectasis was relatively rare (5 of 19 subjects). Improvement in lung function and exercise ability was significantly greater in patients who developed atelectasis. However, they also found improved exercise tolerance in some patients in whom atelectasis did not occur. This may have been due to increased inspiratory capacity or decreased dynamic hyperinflation during exercise. The authors speculated that low collateral resistance in emphysema (40) and incomplete lobar fissures may prevent gas absorption despite airway occlusion, thus limiting the effectiveness of these techniques.
Another bronchoscopic technique seeks to create fenestrations from major airways directly into lung parenchyma. This idea originated from Peter Macklem, who, in 1978, had suggested that the low collateral resistance in emphysema could be exploited by making holes directly through the chest wall into the lung, akin to the spiracles through which insects breathe (41). Lausberg and coworkers showed improved “spirograms” from explanted emphysematous lungs after bronchial fenestration was performed. Increasing the number of fenestrations further improved the spirogram, suggesting significant expiratory flow was occurring through the holes (42).
Few in vivo clinical data have been published on this technique. However, it is apparent that improved expiratory airflow after this procedure in patients is not due to expiration through the new holes. This is because both TLC and RV decrease in subjects who have undergone this procedure (Peter Macklem, M.D., personal communication). If the fenestrations were merely added channels for expiratory flow, RV might decrease but TLC would not. The decrease in both of these lung volumes suggests that the fenestrations allow air trapped in cysts and bullae to slowly drain. Furthermore, these channels must be too small to allow them to refill during a brief inspiration to TLC. Otherwise, TLC would not have fallen. The effect on lung volumes is similar to surgical or other bronchoscopic LVR methods. The implication of this finding is that the size and number of these fenestrations are critical. Too many holes would allow cystic regions to empty and refill during tidal breathing, thus preventing lung volume from falling and increasing dead space ventilation. Clarification of these factors awaits publication of more detailed clinical data.
CONCLUSIONS
LVRS improves lung function primarily by better matching the size of the lungs to the thorax, which contains them. This improves expiratory airflow and reduces dynamic and static hyperinflation. Reduced lung volumes allow the inspiratory muscles to lower pleural pressure with greater mechanical efficiency. Bronchoscopic LVR techniques attempt to achieve the same endpoints without the need for surgery, but the tendency for atelectasis to develop is limited by collateral ventilation. Better understanding of the mechanisms of improvement in lung function may assist in selecting the optimal patients for these procedures.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality (AHRQ).
Conflict of Interest Statement: H.E.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.P.I. is founder and current CSO of Aeris Therapeutics, a biotechnology firm developing bronchoscope methods for achieving lung volume reduction. R.J.M. uses staples produced by Ethicon for LVRS, and he runs a course for minimally invasive lung cancer surgery for which he received $22,500 (10 courses). A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.West JB. Distribution of mechanical stress in the lung, a possible factor in localization of pulmonary disease. Lancet 1971;7704:839–841. [DOI] [PubMed] [Google Scholar]
- 2.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol 2005;98:1892–1899. [DOI] [PubMed] [Google Scholar]
- 3.Stehbens W. Proteinase imbalance versus biomechanical stress in pulmonary emphsyema. Exp Mol Pathol 2000;69:46–62. [DOI] [PubMed] [Google Scholar]
- 4.Fessler HE. Fallingwater and emphysema. Am J Respir Crit Care Med 2001;164:1753–1754. [DOI] [PubMed] [Google Scholar]
- 5.Pride NB, Permutt S, Riley RL, Bromberger-Barnea B. Determinants of maximal expiratory flow from the lungs. J Appl Physiol 1967;23:646–662. [DOI] [PubMed] [Google Scholar]
- 6.Butler J, Caro G, Acala R, DuBois A. Physiological factors affecting airway resistance in normal subjects and in patients with chronic obstructive lung disease. J Clin Invest 1960;43:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 8.Mead J, Lindgren P, Gaensler E. The mechanical properties of the lungs in emphysema. J Clin Invest 1955;34:1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun NM, Arora NS, Rochester DF. Force-length relationship of the normal human diaphragm. J Appl Physiol 1982;53:405–412. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation: the role of lung hyperinflation. Am Rev Respir Dis 1993;148:1351–1357. [DOI] [PubMed] [Google Scholar]
- 11.Fessler HE, Permutt S. Lung volume reduction surgery and airflow limitation. Am J Respir Crit Care Med 1998;157:715–722. [DOI] [PubMed] [Google Scholar]
- 12.Brantigan OC, Mueller E, Kress MB. A surgical approach to pulmonary emphysema. Am Rev Respir Dis 1959;80:194–202. [DOI] [PubMed] [Google Scholar]
- 13.Gelb A, Zamel N, McKenna R, Brenner M. Mechanism of short term improvement in lung function after emphysema resection. Am J Respir Crit Care Med 1996;154:945–951. [DOI] [PubMed] [Google Scholar]
- 14.Gelb AF, McKenna RJ Jr, Brenner M, Fischel R, Baydur A, Zamel N. Contribution of lung and chest wall mechanics following emphysema resection. Chest 1996;110:11–17. [DOI] [PubMed] [Google Scholar]
- 15.Scharf SM, Rossof L, McKeon K, Graver L, Graham C, Steinberg H. Changes in pulmonary mechanics after lung volume reduction surgery. Lung 1998;176:191–204. [DOI] [PubMed] [Google Scholar]
- 16.Ingenito EP, Evans RB, Loring SH, Kaczka DW, Rodenhouse JD, Body SC, Sugarbaker DJ, Mentzer SJ, DeCamp MM, Reilly JJ. Relation between preoperative inspiratory lung resistance and the outcome of lung-volume reduction surgery for emphysema. N Engl J Med 1998;338:1181–1185. [DOI] [PubMed] [Google Scholar]
- 17.Sciurba FC, Rogers RM, Keenan RJ, Slivka W, Gorcsan J, Ferson P, Holbert J, Brown M, Landreneau RJ. Improvement in pulmonary function and elastic recoil after lung reduction surgery for diffuse emphysema. N Engl J Med 1996;334:1095–1099. [DOI] [PubMed] [Google Scholar]
- 18.Criner G, Cordova FC, Leyenson V, Roy B, Travaline J, Sudarshan S, O'Brien G, Kuzma AM, Furukawa S. Effect of lung volume reduction surgery on diaphragm strength. Am J Respir Crit Care Med 1998;157:1578–1585. [DOI] [PubMed] [Google Scholar]
- 19.Ingenito EP, Loring SH, Moy M, Mentzer SJ, Swanson S, Reilly JJ. Interpreting improvement in expiratory flows after lung volume reduction surgery in terms of flow limitation theory. Am J Respir Crit Care Med 2001;163:1074–1080. [DOI] [PubMed] [Google Scholar]
- 20.Fischel RJ, McKenna RJ, Gelb A, Singh N, Brenner M. Insight on emphysema: the first 300 cases of surgical treatment. West J Med 1998;169:74–77. [PMC free article] [PubMed] [Google Scholar]
- 21.McKenna R, Brenner M, Fischel RJ, Singh N, Yoong B, Gelb AF, Osann KE. Patient selection criteria for lung volume reduction surgery. J Thorac Cardiovasc Surg 1997;114:957–967. [DOI] [PubMed] [Google Scholar]
- 22.Ingenito EP, Loring SH, Moy M, Mentzer SJ, Swanson S, Hunsaker A, McKee C, Reilly JJ. Comparison of physiological and radiological screening for lung volume reduction surgery. Am J Respir Crit Care Med 2001;163:1068–1073. [DOI] [PubMed] [Google Scholar]
- 23.National Emphysema Treatment Trial Research Group. A randomized trial comparing lung volume reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 24.Fessler HE, Scharf SM, Permutt S. Improvement in spirometry following lung volume reduction surgery: application of a physiologic model. Am J Respir Crit Care Med 2002;165:34–40. [DOI] [PubMed] [Google Scholar]
- 25.Ingenito EP, Loring SH, Moy M, Mentzer SJ, Swanson S, Reilly JJ. Physiological characterization of variability in response to lung volume reduction surgery. J Appl Physiol 2003;94:20–30. [DOI] [PubMed] [Google Scholar]
- 26.Klineberg P, Rehder K, Hyatt R. Pulmonary mechanics and gas exchange in seated normal men with chest restriction. J Appl Physiol 1981;51:26–32. [DOI] [PubMed] [Google Scholar]
- 27.Teschler H, Stamatis G, Farhat AAE, Meyer FJ, Costabel U, Konietzko N. Effect of surgical lung volume reduction on respiratory muscle function in pulmonary emphysema. Eur Respir J 1996;9:1779–1784. [DOI] [PubMed] [Google Scholar]
- 28.Martinez FJ, Montes de Oca M, Whyte RI, Stetz J, Gay SE, Celli BR. Lung-volume reduction improves dsypnea, dynamic hyperinflation, and respiratory muscle function. Am J Respir Crit Care Med 1997;155:1984–1990. [DOI] [PubMed] [Google Scholar]
- 29.Cassart M, Hamacher J, Verbandt Y, Wildermuth S, Ritscher D, Russi EW, de Francquen P, Cappello M, Weder W, Estenne M. Effects of lung volume reduction surgery for emphysema on diaphragm dimensions and configuration. Am J Respir Crit Care Med 2001;163:1171–1175. [DOI] [PubMed] [Google Scholar]
- 30.Gorman RB, McKenzie DK, Butler LE, Tolman JF, Gandevia SC. Diaphragm length and neural drive after lung volume reduction surgery. Am J Respir Crit Care Med 2005;172:1259–1266. [DOI] [PubMed] [Google Scholar]
- 31.Marchand E, de Leyn P, Gayan-Ramirez G, Palecek F, de Bock V, Dom R, Decramer M. Lung volume reduction surgery does not improve diaphragmatic contractile properties or atrophy in hamsters with elastase-induced emphysema. Am J Respir Crit Care Med 2000;162:1052–1057. [DOI] [PubMed] [Google Scholar]
- 32.Shrager JB, Kim DK, Hashmi YJ, Lankford EB, Wahl P, Stedman HH, Levine S, Kaiser LR. Lung volume reduction surgery restores the normal diaphragmatic length-tension relationship in emphysematous rats. J Thorac Cardiovasc Surg 2001;121:217–224. [DOI] [PubMed] [Google Scholar]
- 33.Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med 1991;325:917–923. [DOI] [PubMed] [Google Scholar]
- 34.Lewis MI, Fournier M, Da XY, Li HM, Mosenifar Z, McKenna RJ, Cohen AH. Short-term influences of lung volume reduction surgery on the diaphragm in emphysematous hamsters. Am J Respir Crit Care Med 2004;170:753–759. [DOI] [PubMed] [Google Scholar]
- 35.Degano B, Brouchet L, Rami J, Arnal JF, Escamilla R, Hermant C, Dahan M. Improvement after lung volume reduction surgery: a role for inspiratory muscle adaptation. Respir Physiolo Neurobiol 2004;139:293–301. [DOI] [PubMed] [Google Scholar]
- 36.Ingenito EP, Berger RL, Henderson AC, Reilly JJ, Tsai L, Hoffman A. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med 2003;167:771–778. [DOI] [PubMed] [Google Scholar]
- 37.Hopkinson NS, Toma TP, Hansell D, Goldstraw P, Moxham J, Geddes D, Polkey MI. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Respir Crit Care Med 2005;171:453–460. [DOI] [PubMed]
- 38.Wan IYP, Toma TP, Geddes DM, Snell G, Williams T, Venuta F, Yim APC. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest 2006;129:518–526. [DOI] [PubMed] [Google Scholar]
- 39.Wood D, McKenna R Jr, Yusen R, Sterman D, Ost D, Springmeyer S, Gonzalez H, Mulligan M, Gildea T, Houck W. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J Thorac Cardiovasc Surg 2007;133:65–73. [DOI] [PubMed] [Google Scholar]
- 40.Terry PB, Traystman RJ, Newball HH, Batra G, Menkes HA. Collateral ventilation in man. N Engl J Med 1978;298:10–15. [DOI] [PubMed] [Google Scholar]
- 41.Macklem PT. Collateral ventilation. N Engl J Med 1978;298:49–50. [DOI] [PubMed] [Google Scholar]
- 42.Lausberg HF, Chino K, Patterson GA, Meyers BF, Toeniskoetter PD, Cooper JD. Bronchial fenestration improves expiratory flow in emphysematous human lungs. Ann Thorac Surg 2003;75:393–397. [DOI] [PubMed] [Google Scholar]
- 43.Ingenito EP, Wood DE, Utz JP. Bronchoscopic lung volume reduction in severe emphysema. Proc Am Thorac Soc 2008;5:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]




