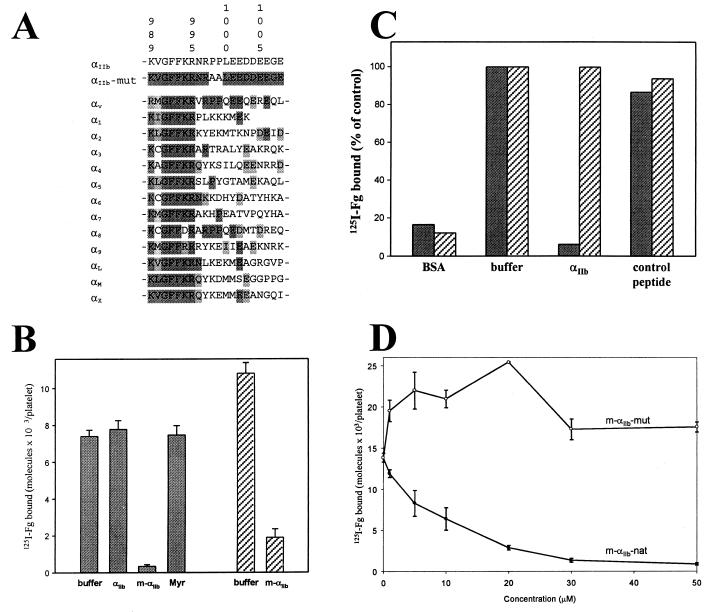
Figure 1.
Sequence and function of the integrin αIIb-cytoplasmic domain. (A) Sequence alignment of various human integrin α-cytoplasmic domains with both wild-type and mutant αIIb. The sequence identity and similarity are highlighted in decreased darkness. Residue numbers correspond to αIIb. (B) Effect of αIIb-cytoplasmic peptides on the binding of fibrinogen (Fg) to stimulated platelets. Human platelets in Tyrode's buffer alone (buffer), with or without 50 μM (final concentration) of nonmyristoylated αIIb K989–E1008 (αIIb), m-αIIb-wt (m-αIIb), and myristic acid (Myr) were preincubated for 30 min with 300 nM 125I-fibrinogen. The platelets were then stimulated with either 10 μM ADP/20 μM epinephrine (shaded bars) or 50 μM thrombin receptor agonist peptide (hatched bars). After 15 min, 125I-fibrinogen binding was measured. Incubation of nonstimulated platelets with cytoplasmic peptides or with myristic acid had no effect on fibrinogen binding (data not shown). (C) Effects of αIIb-cytoplasmic peptides on the binding of fibrinogen to immunocaptured forms of αIIbβ3. Both forms of the receptor, platelet αIIbβ3 (shaded bars) or sr-αIIbβ3 (hatched bars), were immunocaptured onto immobilized monoclonal antibody AP3. The captured receptors were preincubated for 30 min with either buffer alone (buffer) or 50 μM nonmyristoylated αIIb K989–E1008 (αIIb). Peptide specificity was confirmed with a nonrelated acidic peptide (control peptide; GDKNADGWIEFEEL). Results are expressed as a percentage of maximal fibrinogen binding in the absence of peptides. (D) Divergent effects of myristoylated wild-type and mutant αIIb-cytoplasmic peptides on the binding of fibrinogen to stimulated platelets. Platelets were preincubated with varying concentrations of either m-αIIb-wt (closed circles) or m-αIIb-mut peptides (open circles), and then 125I-fibrinogen binding to ADP/epinephrine-stimulated platelets was measured.