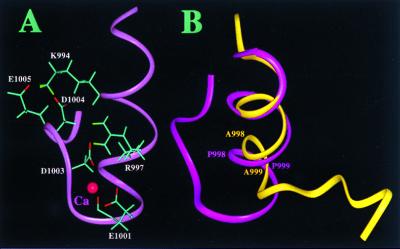
Figure 4.
Structural highlights of the cytoplasmic domain of αIIb. (A) Structure of the m-αIIb-wt showing the side chains of K994, R997, E1001, D1003, D1004, and E1005 for the salt-bridge network. A proposed Ca2+-binding site is also shown in the figure involving R997, E1001, D1003, and D1004, which were identified by comparing the two-dimensional 1H total correlation spectroscopy spectra of Ca2+-free and Ca2+-bound forms (Ca2+ was added up to 5-fold). The binding of Ca2+ to the αIIb-tail has been shown by biochemical studies (12, 27). (B) Backbone overlay of m-αIIb-wt (pink) and m-αIIb-mut (yellow), showing the structural difference. The C-terminal loop of m-αIIb-wt folds back to interact with the N-terminal helix, whereas in the mutant, the C-terminal part is highly flexible and makes no interactions with the helix. The C-terminal part (D1003–E1008) of the m-αIIb-mut is disordered.