Table 3.
Dependence of Ag-catalyzed AVM selectivity on temperaturea
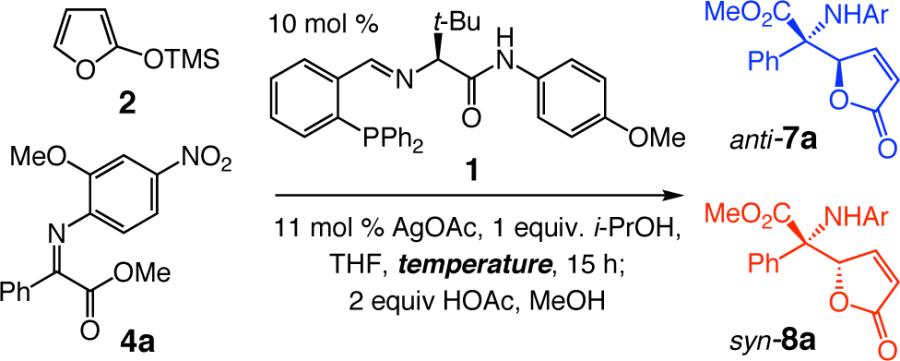 | |||||
|---|---|---|---|---|---|
| entry | temp (°C) | 7a:8ab | yield (%)c | 7a er; ee (%)d | 8a er; ee (%)d |
| 1 | −78 | 93:7 | 88 | 95.5:4.5; 91 | 82.5:17.5; 65 |
| 2 | −50 | 33:66 | 76 | 85:15; 70 | 85:15; 70 |
| 3 | −30 | 14:86 | 58 | 58:42; 16 | 81:19; 62 |
| 4 | −15 | 10:90 | 32 | 63:37; 26 | 79.5:20.5; 59 |
| 5 | +4 | 8:92 | 41 | <55:45; <10 | 77:23; 54 |
Reactions performed under N2 atmosphere; all reactions were quenched at the specified temperature and kept at that temperature for 3 hours before allowing to warm to 22 °C. All conversions = >98% (substrate consumption based on internal standard).
Diastereomeric ratios were determined by 400 MHz 1H NMR analyses of product mixtures prior to purification.
Yields are of purified products; with the exception of entry 1 (yield of only anti-7a), total yields of isomeric mixtures are shown.
Enantiomer ratio (er) values were determined by chiral HPLC analysis; see the Supporting Information for details.
