Abstract
Whole-body hypoxic preconditioning (WHPC) prolongs survival of mice exposed to severe hypoxia by attenuating pulmonary edema and preserving gas exchange. However, the cellular and molecular mechanism(s) of this protection remains unclear. The objective of this study was to identify the cellular target(s) of WHPC in the lung. Conscious mice were exposed to hypoxia (7% O2) for 6 hours with or without pretreatment of WHPC ([8% O2] × 10 min/[21% O2] × 10 min; 6 cycles). Hypoxia caused severe lung injury, as shown by the development of high-permeability–type pulmonary edema and the release of lactate dehydrogenase and creatine kinase into the airspace and the circulation. All these signs of hypoxic lung injury were significantly attenuated by WHPC. Hypoxia also caused a remarkable release of type I cell markers (caveolin-2 and receptor for advanced glycation end products) in lung lavage that was almost completely abolished by WHPC. Conversely, hypoxia-induced release of type II cell markers (surfactant-associated proteins A and D) was only marginal, and was unaffected by WHPC. Electron microscopic analysis demonstrated considerable hypoxic damage in alveolar type I cells and vascular endothelial cells. Notably, WHPC completely eliminated hypoxic damage in the former and alleviated it in the latter. Type II cells appeared normal. Furthermore, WHPC up-regulated protein expression of cytoprotective genes in the lung, such as heat shock proteins and manganese superoxide dismutase. Thus, WHPC attenuates hypoxic lung injury through protection of cells constituting the respiratory membrane, especially hypoxia-vulnerable type I epithelial cells. This beneficial effect may involve up-regulation of cytoprotective genes.
Keywords: pulmonary edema, high-altitude diseases, alveolar fluid clearance, cytoprotection, pulmonary hypertension
CLINICAL RELEVANCE
Type I cells are both the target injury site for hypoxia in the lung and the targeted cell type for protection by whole-body hypoxic preconditioning. This suggests they should be considered as the primary target for therapy, given their newly-defined role in alveolar fluid clearance.
The lung is susceptible to hypoxic injury. Pulmonary edema of the high-permeability type rapidly develops in both human beings and experimental animals subjected to severe hypoxia (1–4). The ensuing thickening of the respiratory membrane and flooding of alveoli reduce gas exchange capacity of the lung and, in turn, further compromise oxygen supply to peripheral tissues, all of which may eventually result in multiple organ failure. Therefore, the lung can be viewed as one of the “rate-limiting” organs in the ability of an organism to survive in severely hypoxic conditions, as evidenced by the fact that high-altitude pulmonary edema is one of the two major causes of death in humans and animals exposed to high-altitude hypoxia (5).
Recent studies indicate that the pathogenesis of hypoxic pulmonary edema involves multiple mechanisms, such as elevated capillary transmural pressure in overperfused lung regions after uneven pulmonary vasoconstriction (6–8), increased vascular permeability secondary to stress failure of the capillary wall (9, 10), and decreased alveolar fluid clearance resulting from functional and/or structural impairment of the alveolar epithelium (11–13). Morphologically, prominent damage to the respiratory membrane that involves disruption of both the vascular endothelium and the alveolar epithelium is evident, and is likely a result of mechanical stress (14, 15) in combination with hypoxic cytotoxicity (10, 16). Experimental therapies targeting these pathological mechanisms have shown promising effects. For example, nitric oxide (NO) gas or phosphodiesterase 5 inhibitors seem to reduce the severity of hypoxic and/or high-altitude pulmonary edema by attenuating uneven vasoconstriction and regional overperfusion (17, 18). Similarly, β-adrenergic receptor agonists have been shown effective in controlling pulmonary edema through several potential mechanisms, including stimulation of the sodium reabsorption machinery in the alveolar epithelium (19–21). However, most of the studies have focused on the restoration of individual cellular functions that are either lost or markedly impaired during hypoxia rather than targeting the hypoxia-induced injury of particularly affected cell populations.
In a previous study, we have shown that whole-body hypoxic preconditioning (WHPC) prolongs the survival of unacclimatized mice exposed to lethal hypoxia, and that this potentially life-saving protective effect is associated with reduced pulmonary edema and improved gas exchange function in the lung and preservation of tissue integrity in other organs, such as the brain (22). We proposed that the organ protection against hypoxic injury afforded by WHPC was conceivably mediated by improved function of “rate-limiting” organs, such as the lung. However, the cellular and molecular mechanism(s) underlying the beneficial effects of WHPC remain entirely unknown. Accordingly, the objective of the current study was to identify the cellular target(s) of WHPC in the lung. Earlier studies using morphological methods seem to suggest that the respiratory membrane is the main site of injury under hypoxic and/or high-altitude conditions (10, 14–16). We therefore hypothesized that WHPC would reduce hypoxia-induced lung injury through preferential protection of cells constituting the respiratory membrane.
MATERIALS AND METHODS
Animals and Hypoxic Exposures
Male C57BL/6 mice, 10 to 12 weeks of age (Taconic Farms, Germantown, NY), were used in this study. All procedures were approved by the Animal Care and Use Committee of the University of Louisville. Animals were randomly divided into three groups: normoxic control, hypoxia, and WHPC + hypoxia. All exposures were conducted in the conscious state. Mice in the WHPC + hypoxia group were subjected to hypoxic preconditioning (6 cycles of 10-min hypoxia [8% O2]/10-min normoxia [room air, 21% O2]) in an environmental chamber (BioSpherix, Inc., Redfield, NY). The ambient CO2 in the chamber was maintained at less than 0.03%, humidity at 40–50%, and temperature at 22–24°C. Mice in the hypoxia group were placed in an adjacent chamber, breathing room air. Two hours after the completion of preconditioning, both groups were exposed to sustained hypoxia (7.0% O2) for 6 hours. Mice in the control group stayed in a room air chamber for the same duration.
Lung Water Content
Mice were killed and thoroughly exsanguinated before their lungs were excised en bloc and blot dried. The wet weight of the tissue was registered immediately with an electronic balance to an accuracy of 0.1 mg. The tissue was then baked in an oven at 65°C under vacuum for 40 hours until a constant weight was achieved. After the dry weight of the tissue was registered, the water content of the tissue was calculated as previously described (22).
Plasma Preparation
After hypoxic exposure in the environmental chamber, mice were immediately anesthetized with Avertin and tracheocannulated within 2 minutes. They were then ventilated with an analyzed gas mixture containing 7.0% O2 and 93% N2 at 110 breaths/minute with a stroke volume of 0.01 ml/g using a rodent ventilator (ASV; Harvard Apparatus, Holliston, MA) for 10 minutes. The heart was then exposed through sternotomy, and blood samples were withdrawn simultaneously from the right and left ventricles. Plasma was collected for analyses after centrifugation of the blood samples at 4,000 × g for 10 minutes.
Bronchoalveolar Lavage Preparation
Bronchoalveolar lavage (BAL) was performed by infusing and withdrawing 1 ml of ice-cold, sterile, normal saline three times through the tracheal cannula. Fluid samples were centrifuged at 2,000 × g for 10 minutes at 4°C, and the supernatant collected for chemistry measurements. The cell pellets from this set of samples were used for cytological analysis. Another set of samples (∼ 500 μl each) was centrifuged at 150,000 × g for 2 hours at 4°C, and the pellet was collected for protein preparation.
Measurement of Albumin
Albumin concentration in the cell-free BAL supernatant was determined using a mouse albumin ELISA quantitation kit (detection range, 7.8–500 ng/ml; Bethyl Laboratories, Montgomery, TX). Measurements were performed in triplicates for each sample following the manufacturer's instructions.
Measurement of Lactate Dehydrogenase and Creatine Kinase Activities
Lactate dehydrogenase (LDH) and creatine kinase (CK) activities in both BAL and plasma samples were determined using a Cobas Mira Plus automated analyzer (Roche Diagnostics Corp., Indianapolis, IN) and LDH and CK assay kits (Pointe Scientific, Inc., Canton, MI). Reagent ratios specified in the manufacturer's manuals were adopted without modification.
Measurement of von Willebrand Factor Antigen
von Willebrand factor antigen (vWF:Ag) levels in plasma samples collected from both the right and the left ventricles were determined using a vWF:Ag ELISA kit (Diagnostica Stago, Inc., Parsippany, NJ). Measurements were performed in triplicate for each sample following the manufacturer's instructions.
Western Blotting Analysis
BAL protein samples were prepared by sonicating the 150,000 × g cell pellet in 80 μl of protein extraction butter containing 50 mM Tris-HCl (pH 7.5), 5 mM EDTA, 10 mM EGTA, 10 mM benzamidine, 50 μg/ml phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, and 10% glycerol (vol/vol) (all chemicals from Sigma-Aldrich, St. Louis, MO). Total cellular protein samples from lung tissues were prepared as described previously (23). Briefly, frozen lung tissues were pulverized and homogenized in the extraction buffer. The homogenate was then incubated on a rocking platform in the presence of 20 mM 3-([3-cholamidopropyl]dimethylammonio)-1-propanesulfonate (Sigma-Aldrich) at 4°C for 4 hours. After centrifugation at 14,000 × g for 30 minutes, the supernatant was collected as total cellular proteins. Protein concentration was determined using a modified Lowry method (DC Protein Assay Kit; Bio-Rad Laboratories, Hercules, CA). Protein samples (20 μl/lane for BAL proteins or 80 μg/lane for total cellular proteins from the lung) were then separated on polyacrylamide/SDS gels and transferred by electroblotting onto a nitrocellulose membrane, which was dyed with reversible Ponceau staining for verification of equal loading and transfer efficiency. After being blocked in 5% nonfat milk, membranes were incubated with a primary antibody (detailed below), followed by incubation with an appropriate horseradish peroxidase–conjugated secondary antibody (Cell Signaling Technology, Danvers, MA). Signal detection was facilitated with enhanced chemiluminescence (ECL kit; GE Lifesciences, Piscataway, NJ). Films were scanned and immunosignals quantitated using ImageQuant software v5.2 (Molecular Dynamics/GE Lifesciences).
Primary Antibodies and Their Sources
The following primary antibodies were used in the study: caveolin-2 antibody (cat. no. 610,685; BD Pharmingen, San Diego, CA); receptor for advanced glycation end products (RAGE) antibody (cat. no. AF1179; R&D Systems, Inc., Minneapolis, MN); surfactant-associated protein (SP)-A and SP-D (cat. nos. AB3420 and AB3434, respectively), heat shock protein (HSP) 32 (cat. no. AB1284), manganese superoxide dismutase (MnSOD) (cat. no. 06–984), copper–zinc SOD (cat. no. 06–482), and inducible NO synthase (cat. no. 06,573) antibodies were from Millipore (Temecula, CA); HSP27 and HSP70 antibodies (cat. no. sc-1048 and cat. no. sc-24, respectively; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); and cyclooxygenase-2 antibody (cat. no. 160,106; Cayman Chemical, Ann Arbor, MI).
Cytological Analysis of BAL Samples
Cell pellets used for this analysis were obtained by centrifugation of BAL samples at 2,000 × g for 10 minutes at 4°C. Cell pellets were thoroughly resuspended in normal saline and then transferred to a glass slide through a cytofunnel (Thermoshandon, Pittsburgh, PA) by centrifugation at 1,500 rpm for 2 minutes. The cytospin slides were fixed in 95% alcohol for 10 minutes and Papanicolaou stained for microscopic analysis by a qualified pathologist.
Transmission Electron Microscopy
Mouse lungs were inflation fixed at 25 cm H2O with 2.5% glutaraldehyde in PBS. Lungs were removed and immersed in the same fixative overnight at 4°C. Several 1-mm3 cubes were removed from the lungs, washed three times in PBS, and post-fixed in aqueous 1% OsO4/1% K3Fe(CN)6 for 1 hour. After three PBS washes, the pellet was dehydrated through a graded series of 30–100% ethanol/100% propylene oxide, and then infiltrated in 1:1 mixture of propylene oxide:Polybed 812 epoxy resin (Polysciences, Warrington, PA) for 1 hour. After several changes of 100% resin over 24 hours, the pellet was embedded in molds and cured at 37°C overnight, followed by additional hardening at 65°C for 2 more days. Ultrathin (60-nm) sections were collected on 200-mesh copper grids and stained with 2% uranyl acetate in 50% methanol for 10 minutes, followed by 1% lead citrate for 7 minutes. Sections were photographed using a JEM 1,210 transmission electron microscope (JEOL, Peabody, MA) equipped with a CCD camera (Advanced Microscopy Techniques Corp., Danvers, MA) at 80 kV.
Statistical Analyses
Comparisons among groups were conducted using one-way ANOVA followed by unpaired Student's t tests with the Bonferroni correction. Comparisons between the control and the WHPC groups were made using unpaired Student's t tests. A P value less than 0.05 was considered statistically significant.
RESULTS
WHPC Attenuated, Hypoxia-Induced Lung Injury
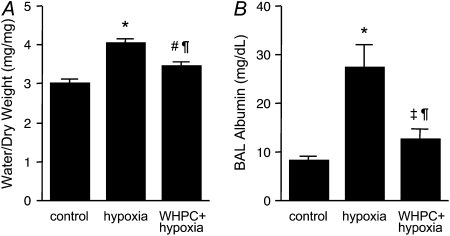
Naive mice developed pulmonary edema after being exposed to severe hypoxia (FiO2 = 0.07) for 6 hours, as shown by increased water content in lung tissues (Figure 1A). This was accompanied by an increase in pulmonary vascular permeability, as indicated by substantially elevated albumin levels in the BAL fluid from these animals (Figure 1B). Both of these pathological changes, however, were significantly attenuated in WHPC-treated mice (Figure 1).
Figure 1.
Whole-body hypoxic preconditioning (WHPC) attenuates hypoxic pulmonary edema and hypoxia-induced increase in pulmonary vascular permeability. Exposure to hypoxia for 6 hours resulted in (A) an increase in lung tissue water content (n = 8 for each group) and (B) elevated levels of albumin in bronchoalveolar lavage (BAL) (n = 10 for each group). Both of these hypoxia-induced pathological changes were attenuated by pretreatment with WHPC. All data are mean ± SE. *P < 0.001 versus control; #P < 0.005 versus control; ‡P < 0.05 versus control; ¶P < 0.005 versus hypoxia.
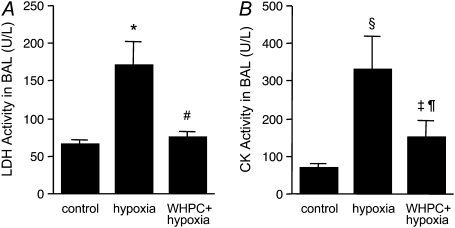
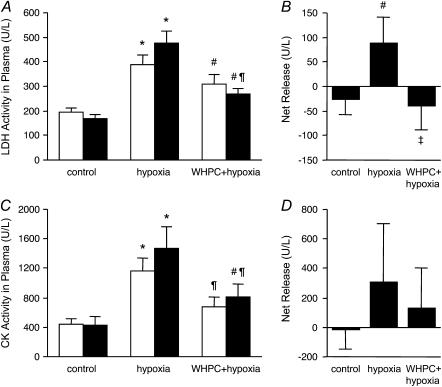
To determine whether these beneficial effects of WHPC were derived from preservation of lung cellular integrity, we measured general cellular injury markers released by the lung toward both the airspace and the circulation. We found that hypoxia resulted in conspicuous cellular injury in the lung, as evidenced by substantial increases in both LDH and CK activities in the BAL fluid in naive mice (Figure 2). Such hypoxia-induced cellular injury was greatly diminished by WHPC, as the increase in LDH activities after hypoxic exposure was completely abolished, whereas that in CK activities was significantly reduced, in WHPC-treated animals (Figure 2). Hypoxia also elicited an increase in LDH activities in blood samples collected from both the right and the left ventricles; however, only those in blood from the left ventricle were significantly reduced by WHPC (Figure 3A). Because LDH activities in blood from the left ventricle reflected a combination of those derived from peripheral tissues and from the lung, net release of LDH activities from the lung in each animal was calculated by subtracting the right ventricle value from the left ventricle value so that occurrence and prevention of cellular injury in the lung could be more accurately assessed. Indeed, hypoxia led to a net release of LDH activities from the lung in naive mice, which was completely abolished in WHPC-treated animals (Figure 3B). Meanwhile, hypoxia caused a similar increase in CK activities in blood samples collected from both the right and the left ventricles, both of which were significantly reduced in WHPC-treated animals (Figure 3C). Because the net releases of this injury marker from the lung among the three experimental groups were not significantly different (Figure 3D), both hypoxia-caused cellular injury and WHPC-afforded protection, as shown by this marker, probably reflected changes in peripheral tissues other than the lung.
Figure 2.
WHPC reduces hypoxic lung injury. Exposure to hypoxia for 6 hours caused severe lung injury, as indicated by elevated levels of general injury markers, lactate dehydrogenase (LDH) (A) and creatine kinase (CK) (B) in BAL. Pretreatment with WHPC effectively abolished the hypoxia-induced release of LDH, and significantly reduced that of CK in BAL. All data are means ± SE; n = 10 for each group. *P < 0.005 versus control; #P < 0.005 versus hypoxia; §P < 0.01 versus control; ‡P < 0.05 versus control; ¶P < 0.05 versus hypoxia.
Figure 3.
WHPC reduces hypoxia-induced release of injury markers into the pulmonary circulation. The differences between LDH and CK activities in blood samples collected from the left ventricle (filled bars, pulmonary efferent blood) and those in blood samples collected from the right ventricle (open bars, pulmonary afferent blood) were calculated and taken as the net release of these injury markers from the lung. (A and B) Exposure to hypoxia increased the release of LDH from both peripheral tissues and the lung. Pretreatment with WHPC significantly diminished LDH release from the lung, but had little effect on that from peripheral tissues. *P < 0.001 versus control; #P < 0.05 versus control; ¶P < 0.001 versus hypoxia; ‡P < 0.05 versus hypoxia. (C and D) Exposure to hypoxia increased the release of CK, mainly from peripheral tissues, which was significantly attenuated by pretreatment with WHPC. *P < 0.005 versus control; #P < 0.05 versus control; ¶P < 0.05 versus hypoxia. All data are means ± SE; n = 10 for each group.
WHPC Preferentially Protected Type I Epithelial Cells
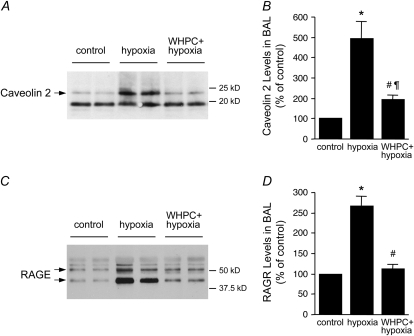
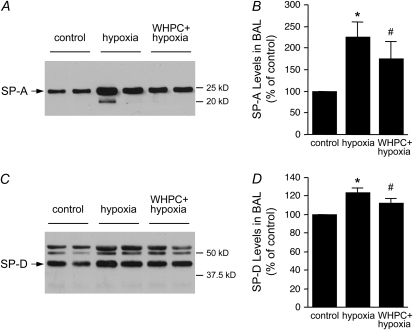
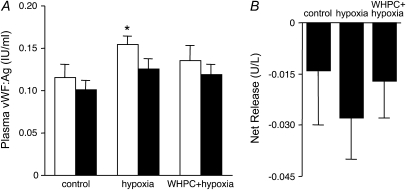
To define the cellular target(s) of hypoxic lung injury and, in turn, the target(s) of WHPC-afforded protection, we examined release of cell type–specific injury markers in BAL and blood samples. Specifically, caveolin-2 (24) and RAGE (25) were measured as injury markers for type I epithelial cells, SP-A and SP-D for type II epithelial cells (26), and vWF:Ag for vascular endothelial cells (27). We found that hypoxia resulted in significant type I epithelial cell damage in naive mice, as shown by the remarkable increase in caveolin-2 and RAGE protein levels in the BAL fluid in these animals (Figure 4). This hypoxia-induced cellular injury was effectively prevented by WHPC, as RAGE protein levels in the BAL fluid in these treated animals were similar to those in normoxic control animals, whereas caveolin-2 protein levels were only modestly higher than those in normoxic control animals, but substantially lower than those in naive animals exposed to hypoxia (Figure 4). On the other hand, the injurious effect of hypoxia on type II epithelial cells seemed to be mild, as hypoxia-induced increase in SP-A and SP-D protein levels in the BAL fluid of naive mice was relatively minor. WHPC did not significantly modify the hypoxia-induced minor increase in these two type II epithelial markers (Figure 5). Surprisingly, hypoxia caused only a small increase in vWF:Ag levels in blood collected from the right ventricle, whereas it had no significant effect on those from the left ventricle (Figure 6A). There was no net release of vWF:Ag into the pulmonary circulation under any of the experimental conditions (Figure 6B). These results were in contrast with the well documented injurious effect of hypoxia on the pulmonary vascular endothelium. To determine whether vWF:Ag was released toward the interstitium because of the direction of fluid flux in hypoxic lungs, additional experiments were performed to measure levels of this injury marker in BAL samples. We found that levels of this marker were very low in the BAL fluid, and that there were no differences among the three experimental groups (data not shown).
Figure 4.
WHPC minimizes hypoxia-induced release of type I epithelial markers. (A) Exposure to hypoxia caused a substantial increase in caveolin-2 protein levels in BAL, which was greatly attenuated by pretreatment with WHPC. Shown is a representative Western blot. (B) Densitometric analysis of caveolin-2 immunosignals from five blots. Data are means ± SE; n = 10 for each group. *P < 0.001 versus control; #P < 0.005 versus control; ¶P < 0.01 versus hypoxia. (C) Hypoxia exposure also increased receptor for advanced glycation end products (RAGE) levels in BAL, which was completely abolished by pretreatment with WHPC. Shown is a representative Western blot. (D) Densitometric analysis of RAGE immunosignals from 4 blots. Data are mean ± SE; n = 8 for each group. *P < 0.001 versus control; #P < 0.001 versus hypoxia.
Figure 5.
Effects of hypoxia and WHPC on the release of type II epithelial markers. (A) Exposure to hypoxia caused a small increase in surfactant-associated protein (SP)-A levels in BAL. However, pretreatment with WHPC had little effect on this hypoxia-induced pathological change. Shown is a representative Western blot. (B) Densitometric analysis of SP-A immunosignals from four blots. Data are means ± SE; n = 8 for each group. *P < 0.005 versus control; #P < 0.05 versus control. (C) Hypoxia exposure caused a marginal increase in SP-D levels in BAL, which remained unchanged with WHPC pretreatment. Shown is a representative Western blot. (D) Densitometric analysis of SP-D immunosignals from four blots. Data are means ± SE; n = 8 for each group. *P < 0.001 versus control; #P < 0.05 versus control.
Figure 6.
Effects of hypoxia and WHPC on the release of an endothelial marker. The difference between von Willebrand factor antigen (vWF:Ag) levels in blood samples collected from the left ventricle (filled bars, pulmonary efferent blood) and those in blood samples collected from the right ventricle (open bars, pulmonary afferent blood) was calculated and taken as the net release of this endothelium-specific marker from the pulmonary vasculature. (A) Exposure to hypoxia caused a minor increase in vWF:Ag release from the peripheral, but not the pulmonary vasculature. The former was moderated by pretreatment with WHPC. *P < 0.05 versus control. (B) There did not appear to be a net release of vWF:Ag from the pulmonary vasculature under any of the conditions tested. Data are means ± SE; n = 16 for each group.
BAL samples from all three groups of mice were also subjected to cytological analysis. BAL from normoxic control mice contained primarily macrophages, with rare ciliated and nonciliated epithelial cells. A small amount of red blood cells was also seen in some samples. BAL samples from naive mice exposed to hypoxia, however, demonstrated a significantly increased number of ciliated and nonciliated epithelial cells and red blood cells. The ratio of epithelial cells to macrophages was evidently elevated from 1:10 to 1:2 on average, with the highest ratio close to 1:1. These BAL samples also contained amorphous proteinaceous materials, most likely representing alveolar edema. There was no evidence of increased inflammatory cells, including neutrophils and lymphocytes. These hypoxia-induced pathological changes in the BAL fluid, however, were almost completely absent in mice pretreated with WHPC. The cytological pattern of the BAL fluid from the WHPC group was undistinguishable from that of normoxic control animals.
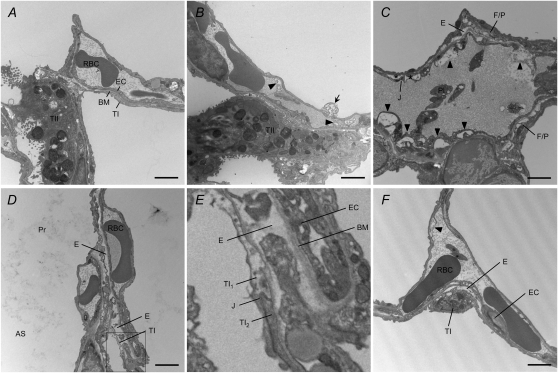
Hypoxia-induced lung injury and the protective effect of WHPC were further assessed with electron microscopy. Considerable damage to type I epithelial cells, endothelial cells, interstitial fibroblasts, and pericytes was observed in lungs of naive mice exposed to hypoxia. The types of cellular injury included necrosis, blebs, detachment from the basement membrane, and intracellular edema. Alveolar edema, interstitial edema, especially where epithelial and endothelial basement membrane were separated by interstitial fibroblasts or pericytes, and disappearance/disruption of basement membrane were also commonly observed. However, damage to type II epithelial cells was rare, if present at all, and alveolar macrophages looked normal. As reported in the literature (1–2), hypoxic lung injury was noticeably uneven. An area with substantial damage could be neighbored by areas seemingly normal, and vice versa. In lungs of hypoxic mice pretreated with WHPC, no visible signs of injury in type I epithelial cells were observed in any areas examined, and there was hardly any alveolar edema. Minor injury to endothelial cells, fibroblasts, and pericytes, such as small blebs, and minor interstitial edema scattered in limited areas. Representative micrographs are presented in Figure 7.
Figure 7.
Electron microscopic analysis of hypoxic lungs with or without WHPC. (A) Parenchymal ultrastructure of a normoxic lung showing a capillary lined with endothelial cells (EC) and alveolar space lined with type I epithelial cells (TI). Both EC and TI are attached to a thin basement membrane (BM). An adjacent type II epithelial cell (TII) is also shown. RBC = red blood cell. (B) Hypoxia caused significant injury in both EC and TI, whereas TII looked relatively normal. Blebs in EC (arrow head) and TI (arrow) are shown. (C) A severely injured blood vessel characterized by blebs in EC (arrow head) and EC peeling off BM (asterisks). A tight junction (J) between peeled edges of EC appeared intact. Also shown are interstitial edema (E) and damaged interstitial fibroblasts/pericytes (F/P). Pl = platelets. (D) Hypoxic pulmonary edema occurred in both the air space (AS), as shown by proteinaceous materials (Pr), and the interstitium, as shown by enlarged interstitial space. A necrotic TI is also shown. (E) Enlargement of the boxed area from (D) showing a necrotic type I cell (TI1). The neighboring type I cell (TI2) and the tight junction, as well as the adjacent vessel, appeared relatively normal. (F) WHPC effectively limited hypoxic lung injury. Minor EC blebs and interstitial edema were the only signs of injury, whereas epithelial cells looked normal. Bars = 2 μm.
WHPC Up-Regulated Cytoprotective Genes in the Lung
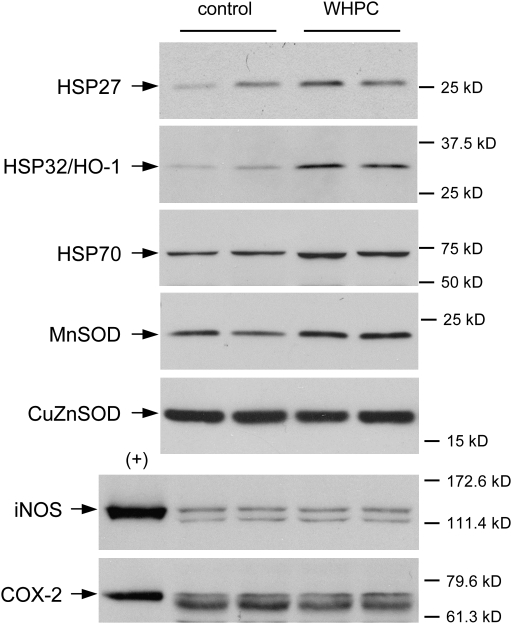
To investigate potential mechanisms that may underlie WHPC-afforded protection against hypoxia, we examined lung expression patterns of several genes, which were known to mediate cytoprotection against ischemia-/hypoxia-induced injury in various organs. We found that WHPC up-regulated the expression of several members of the HSP gene family in the lung. In particular, protein levels of HSP27, HSP32 (also known as heme oxygenase-1, or HO-1), and HSP70 in the lung of WHPC-treated mice were substantially elevated in comparison with those in the lung of normoxic control animals (Figure 8). WHPC also up-regulated the protein expression of MnSOD, but not copper–zinc SOD, in the lung (Figure 8). However, WHPC did not appear to affect the expression patterns of inducible NO synthase or cyclooxygenase-2, which were previously shown to mediate the protection afforded by ischemic preconditioning in the heart (28, 29).
Figure 8.
WHPC up-regulates protein expression of cytoprotective genes in the lung. Lung protein samples were isolated from mice 2 hours after WHPC treatment. Kidney protein from a lipopolysaccharide-treated rat was used as positive controls (+) for inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2. Shown are representative Western blots. CuZnSOD = copper–zinc superoxide dismutase; HSP = heat shock protein; MnSOD = manganese superoxide dismutase.
DISCUSSION
The current study demonstrates that both type I epithelial cells and vascular endothelial cells are highly vulnerable to hypoxic injury and are targets of WHPC-afforded protection. Considering that these two cell types are the main cellular populations constituting the respiratory membrane, preservation of their integrity is conceivably a critical component of the overall protection against hypoxia afforded by WHPC. Furthermore, WHPC-afforded protection against hypoxia appears to be most prominent in type I epithelial cells, as shown by the total or near-total disappearance of signs of hypoxic injury in these cells, including the release of biochemical markers, shed cells in the BAL fluid, and morphological damage in the lung of WHPC-treated animals.
The lung epithelium plays important roles in regulating fluid and solute exchange in the lung and, ultimately, in moderating the development of hypoxic pulmonary edema (30–32). For example, the structural integrity of the lung epithelium serves as a physical barrier that impedes fluid flux from the interstitium to alveoli, which in turn prevents fluid filtration from the pulmonary circulation into the interstitium. Disruption of the epithelial lining, or even impairment of tight junctions between individual epithelial cells, will result in a free passage of fluid and macromolecules into alveoli (alveolar flooding) and, in turn, promote fluid filtration from the circulation (12, 14). Our observation that preservation of the structural integrity of the lung epithelial lining is associated with reduced hypoxic pulmonary edema in WHPC-treated animals is in agreement with this notion. Furthermore, the alveolar epithelium is fundamentally important in fluid clearance from the distal airspace in the lung through its sodium and water reabsorption functions that involve a coordinated operation of the sodium channel in the apical membrane, the Na,K-ATPase in the basolateral membrane, and aquaporin water channels (30–32). Hypoxia has been shown to inhibit the sodium channel and Na,K-ATPase at the activity and/or the expression levels, and to reduce transepithelial sodium flux and, eventually, alveolar fluid clearance (13, 33–36). Such impairment of the epithelial reabsorption function is considered a key factor in the development of hypoxic pulmonary edema (30, 33). It is possible that WHPC also acts to preserve this important function of the alveolar epithelium through up-regulation of HSP32/HO-1 and MnSOD (see below).
It must be pointed out that sodium reabsorption is traditionally regarded as a function of type II epithelial cells; however, recent studies have shown that type I epithelial cells contain sodium transport proteins and transport activities, both of which are expressed at levels higher than those observed in type II cells (37, 38). Considering that type I cells cover more than 95% of the alveolar surface area, it is likely that these cells play a major role in sodium and water reabsorption in the lung. In the current study, we show that hypoxic injury to alveolar type I epithelial cells is associated with alveolar edema, whereas WHPC affords effective protection of these cells, and is associated with avoidance of hypoxic alveolar edema. These findings are therefore consistent with a new paradigm, whereby type I epithelial cells are both the target injury site for hypoxia in the lung, and the targeted cell type for protection by WHPC.
Given the heterogeneity and uneven nature of hypoxic lung injury, the use of biochemical markers, especially those with relative cell type specificities, represents a valuable alternative for overall quantitative assessments of the severity of the injury and the effectiveness of therapeutic interventions. Of the cell type–specific markers used in the current study, caveolin-2 (24) and RAGE (25) proved to be reasonably reliable markers for hypoxia-induced injury in type I epithelial cells, as their release from the lung was well correlated with the status of these cells, as evaluated with conventional morphological approaches. Release patterns of SP-A and SP-D under hypoxic conditions were generally consistent with the status of type II epithelial cells. The mild increase of these markers in the BAL fluid from hypoxic mice in the absence of morphological damage in type II epithelial cells could be attributed to increased secretion of surfactant by mechanical and/or biochemical stimuli (39, 40), including physical stretch and hormonal changes that are associated with hypoxic exposure. Interestingly, increased secretion of surfactant has been linked to improved outcomes in several types of acute lung injury (41–43). A major discrepancy, however, exists between the presence of hypoxia-induced injury in the pulmonary vascular endothelium, as shown by the electron microscopic analysis, and the absence of hypoxia-induced vWF:Ag release into the pulmonary circulation in the current study. vWF:Ag has been shown to be a sensitive marker for endothelial damage, and has been used for the diagnosis and prognosis of several types of acute lung injury in humans (27, 44, 45) and in rats (46). The failure of this marker in reflecting hypoxia-induced injury in pulmonary endothelial cells in the current study was therefore unexpected. Although the ELISA kit was based on a rabbit anti-human vWF polyclonal antibody, lack of cross-species reactivity was unlikely the reason, because the same antibody worked well with the rat vWF (46), which shared greater than 96% similarity with the mouse protein. A possible explanation lies in the heterogeneous expression pattern of vWF in the pulmonary vasculature. Several studies have found that vWF is weakly expressed in endothelial cells of alveolar wall capillaries, and that its expression levels in pulmonary vascular endothelial cells increase gradually with vessel caliber (47, 48). Given that hypoxic injury in the pulmonary circulation affects mainly capillaries and small-resistance vessels, it certainly can be questioned whether vWF is a sensitive marker for this particular type of endothelial injury.
The molecular nature of the WHPC-afforded protection against hypoxic lung injury has yet to be elucidated. Results from the current study, however, seem to indicate that WHPC, at least in part, acts to enhance the resistance of susceptible cell populations to hypoxic insult through selective up-regulation of cytoprotective genes. Indeed, protein levels of HSP27, HSP32/HO-1, and HSP70 rapidly increased in the lung after WHPC treatment. Given the well documented cytoprotective effects of these HSPs against various types of stress, including hypoxia (49, 50), it is reasonable to assume that their up-regulation would at least partially account for the WHPC-afforded protection against hypoxic injury in the lung. Furthermore, recent studies demonstrated that hypoxia caused endocytosis and inhibition of Na,K-ATPase in alveolar epithelial cells through increased production of reactive oxygen species in mitochondria (11), and that overexpression of a mitochondrial antioxidant enzyme, MnSOD, prevented these adverse effects (21). These interesting findings raise the possibility that HSP32/HO-1, an important antioxidant enzyme, may act in concert with MnSOD, which was also up-regulated in the lung by WHPC, to prevent hypoxia-induced endocytosis and inhibition of Na,K-ATPase and, thus, preserve fluid reabsorption functions of the alveolar epithelium. This is particularly relevant when one considers that HSP32/HO-1 is highly expressed in the lung epithelium (51), and has been shown to localize to the mitochondrion of alveolar epithelial cells (52).
In summary, WHPC attenuates hypoxic pulmonary edema and hypoxia-induced increase in pulmonary vascular permeability through protection of cells constituting the respiratory membrane, especially hypoxia-vulnerable type I epithelial cells. We postulate that this beneficial effect may involve up-regulation of cytoprotective genes that preserve the structural and functional integrity of protected cells.
Acknowledgments
The authors thank Dr. Shangzhi Guo for his assistance in surgical procedures.
This work was supported by National Heart, Lung, and Blood Institute grant R01HL074369 (Y.W.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0003OC on September 5, 2008
Conflict of Interest Statement: D.G. serves on the National Speaker's Bureau for Merck Company and has received lecture honoraria totaling $12,000 for 2007. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bartsch P, Mairbaurl H, Maggiorini M, Swenson ER. Physiological aspects of high-altitude pulmonary edema. J Appl Physiol 2005;98:1101–1110. [DOI] [PubMed] [Google Scholar]
- 2.Sartori C, Allemann Y, Scherrer U. Pathogenesis of pulmonary edema: learning from high-altitude pulmonary edema. Respir Physiol Neurobiol 2007;159:338–349. [DOI] [PubMed] [Google Scholar]
- 3.Staub NC. Pulmonary edema. Physiol Rev 1974;54:678–811. [DOI] [PubMed] [Google Scholar]
- 4.Weir EK, Reeves JT. Pulmonary edema. Armonk, NY: Futura Pub. Co.; 1998.
- 5.Gallagher SA, Hackett PH. High-altitude illness. Emerg Med Clin North Am 2004;22:329–355, viii. [DOI] [PubMed] [Google Scholar]
- 6.Dehnert C, Risse F, Ley S, Kuder TA, Buhmann R, Puderbach M, Menold E, Mereles D, Kauczor HU, Bartsch P, et al. Magnetic resonance imaging of uneven pulmonary perfusion in hypoxia in humans. Am J Respir Crit Care Med 2006;174:1132–1138. [DOI] [PubMed] [Google Scholar]
- 7.Hlastala MP, Lamm WJ, Karp A, Polissar NL, Starr IR, Glenny RW. Spatial distribution of hypoxic pulmonary vasoconstriction in the supine pig. J Appl Physiol 2004;96:1589–1599. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins SR, Garg J, Bolar DS, Balouch J, Levin DL. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am J Respir Crit Care Med 2005;171:83–87. [DOI] [PubMed] [Google Scholar]
- 9.Tsukimoto K, Mathieu-Costello O, Prediletto R, Elliott AR, West JB. Ultrastructural appearances of pulmonary capillaries at high transmural pressures. J Appl Physiol 1991;71:573–582. [DOI] [PubMed] [Google Scholar]
- 10.West JB, Colice GL, Lee YJ, Namba Y, Kurdak SS, Fu Z, Ou LC, Mathieu-Costello O. Pathogenesis of high-altitude pulmonary oedema: direct evidence of stress failure of pulmonary capillaries. Eur Respir J 1995;8:523–529. [PubMed] [Google Scholar]
- 11.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest 2003;111:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 1990;142:1250–1257. [DOI] [PubMed] [Google Scholar]
- 13.Planes C, Escoubet B, Blot-Chabaud M, Friedlander G, Farman N, Clerici C. Hypoxia downregulates expression and activity of epithelial sodium channels in rat alveolar epithelial cells. Am J Respir Cell Mol Biol 1997;17:508–518. [DOI] [PubMed] [Google Scholar]
- 14.Bachofen H, Schurch S, Weibel ER. Experimental hydrostatic pulmonary edema in rabbit lungs: barrier lesions. Am Rev Respir Dis 1993;147:997–1004. [DOI] [PubMed] [Google Scholar]
- 15.Costello ML, Mathieu-Costello O, West JB. Stress failure of alveolar epithelial cells studied by scanning electron microscopy. Am Rev Respir Dis 1992;145:1446–1455. [DOI] [PubMed] [Google Scholar]
- 16.Sobin SS, Chen PC. Ultrastructural changes in the pulmonary arterioles in acute hypoxic pulmonary hypertension in the rat. High Alt Med Biol 2000;1:311–322. [DOI] [PubMed] [Google Scholar]
- 17.Nagamine J, Hill LL, Pearl RG. Combined therapy with zaprinast and inhaled nitric oxide abolishes hypoxic pulmonary hypertension. Crit Care Med 2000;28:2420–2424. [DOI] [PubMed] [Google Scholar]
- 18.Scherrer U, Vollenweider L, Delabays A, Savcic M, Eichenberger U, Kleger GR, Fikrle A, Ballmer PE, Nicod P, Bartsch P. Inhaled nitric oxide for high-altitude pulmonary edema. N Engl J Med 1996;334:624–629. [DOI] [PubMed] [Google Scholar]
- 19.Sartori C, Allemann Y, Duplain H, Lepori M, Egli M, Lipp E, Hutter D, Turini P, Hugli O, Cook S, et al. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med 2002;346:1631–1636. [DOI] [PubMed] [Google Scholar]
- 20.Bärtsch P, Mairbäurl H. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med 2002;347:1282–1285. [PubMed] [Google Scholar]
- 21.Litvan J, Briva A, Wilson MS, Budinger GR, Sznajder JI, Ridge KM. β-adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na,K-ATPase and alveolar fluid reabsorption. J Biol Chem 2006;281:19892–19898. [DOI] [PubMed] [Google Scholar]
- 22.Zhang SX, Miller JJ, Gozal D, Wang Y. Whole-body hypoxic preconditioning protects mice against acute hypoxia by improving lung function. J Appl Physiol 2004;96:392–397. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SX, Searcy TR, Wu Y, Gozal D, Wang Y. Alternative promoter usage and alternative splicing contribute to mRNA heterogeneity of mouse monocarboxylate transporter 2. Physiol Genomics 2007;32:95–104. [DOI] [PubMed] [Google Scholar]
- 24.Krasteva G, Pfeil U, Drab M, Kummer W, Konig P. Caveolin-1 and -2 in airway epithelium: expression and in situ association as detected by FRET-CLSM. Respir Res 2006;7:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 2006;173:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle IR, Bersten AD, Nicholas TE. Surfactant proteins-A and -B are elevated in plasma of patients with acute respiratory failure. Am J Respir Crit Care Med 1997;156:1217–1229. [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB, Wiener-Kronish JP, Murray JF, Green DR, Turner J, Luce JM, Montgomery AB, Marks JD, Matthay MA. Elevated von willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest 1990;86:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Guo Y, Zhang SX, Wu WJ, Wang J, Bao W, Bolli R. Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol 2002;34:5–15. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Kodani E, Wang J, Zhang SX, Takano H, Tang XL, Bolli R. Cardioprotection during the final stage of the late phase of ischemic preconditioning is mediated by neuronal no synthase in concert with cyclooxygenase-2. Circ Res 2004;95:84–91. [DOI] [PubMed] [Google Scholar]
- 30.Hoschele S, Mairbaurl H. Alveolar flooding at high altitude: failure of reabsorption? News Physiol Sci 2003;18:55–59. [DOI] [PubMed] [Google Scholar]
- 31.Jain M, Sznajder JI. Effects of hypoxia on the alveolar epithelium. Proc Am Thorac Soc 2005;2:202–205. [DOI] [PubMed] [Google Scholar]
- 32.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600. [DOI] [PubMed] [Google Scholar]
- 33.Hardiman KM, Matalon S. Modification of sodium transport and alveolar fluid clearance by hypoxia: mechanisms and physiological implications. Am J Respir Cell Mol Biol 2001;25:538–541. [DOI] [PubMed] [Google Scholar]
- 34.Vivona ML, Matthay M, Chabaud MB, Friedlander G, Clerici C. Hypoxia reduces alveolar epithelial sodium and fluid transport in rats: reversal by β-adrenergic agonist treatment. Am J Respir Cell Mol Biol 2001;25:554–561. [DOI] [PubMed] [Google Scholar]
- 35.Thome UH, Davis IC, Nguyen SV, Shelton BJ, Matalon S. Modulation of sodium transport in fetal alveolar epithelial cells by oxygen and corticosterone. Am J Physiol Lung Cell Mol Physiol 2003;284:L376–L385. [DOI] [PubMed] [Google Scholar]
- 36.Wodopia R, Ko HS, Billian J, Wiesner R, Bartsch P, Mairbaurl H. Hypoxia decreases proteins involved in epithelial electrolyte transport in A549 cells and rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L1110–L1119. [DOI] [PubMed] [Google Scholar]
- 37.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 2006;103:4964–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci USA 2002;99:1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mason RJ, Voelker DR. Regulatory mechanisms of surfactant secretion. Biochim Biophys Acta 1998;1408:226–240. [DOI] [PubMed] [Google Scholar]
- 40.Wright JR, Dobbs LG. Regulation of pulmonary surfactant secretion and clearance. Annu Rev Physiol 1991;53:395–414. [DOI] [PubMed] [Google Scholar]
- 41.Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T, Kuroki Y, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 1999;160:1843–1850. [DOI] [PubMed] [Google Scholar]
- 42.Griese M, Maderlechner N, Ahrens P, Kitz R. Surfactant proteins A and D in children with pulmonary disease due to gastroesophageal reflux. Am J Respir Crit Care Med 2002;165:1546–1550. [DOI] [PubMed] [Google Scholar]
- 43.Baker RR, Holm BA, Panus PC, Matalon S. Development of O2 tolerance in rabbits with no increase in antioxidant enzymes. J Appl Physiol 1989;66:1679–1684. [DOI] [PubMed] [Google Scholar]
- 44.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med 2004;170:766–772. [DOI] [PubMed] [Google Scholar]
- 45.Flori HR, Ware LB, Milet M, Matthay MA. Early elevation of plasma von Willebrand factor antigen in pediatric acute lung injury is associated with an increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med 2007;8:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med 2002;165:242–249. [DOI] [PubMed] [Google Scholar]
- 47.Muller AM, Skrzynski C, Skipka G, Muller KM. Expression of von Willebrand factor by human pulmonary endothelial cells in vivo. Respiration 2002;69:526–533. [DOI] [PubMed] [Google Scholar]
- 48.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and FLI-1 in normal human tissues. J Histochem Cytochem 2006;54:385–395. [DOI] [PubMed] [Google Scholar]
- 49.Heads RJ, Yellon DM, Latchman DS. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol 1995;27:1669–1678. [DOI] [PubMed] [Google Scholar]
- 50.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev 2006;86:583–650. [DOI] [PubMed] [Google Scholar]
- 51.Cardell LO, Lou YP, Takeyama K, Ueki IF, Lausier J, Nadel JA. Carbon monoxide, a cyclic GMP–related messenger, involved in hypoxic bronchodilation in vivo. Pulm Pharmacol Ther 1998;11:309–315. [DOI] [PubMed] [Google Scholar]
- 52.Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, et al. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke–induced cell death. Am J Respir Cell Mol Biol 2007;36:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]