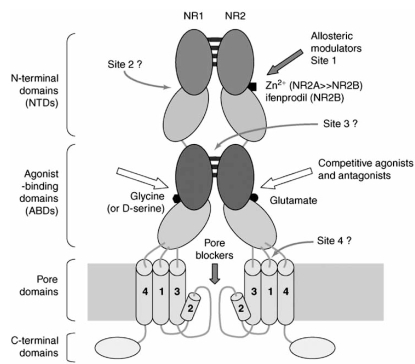
Fig. (3).Potential sites for ligand binding at NMDARs.
Most NMDAR are believed to assemble as tetramers, associating two NR1 and two NR2 subunits in a ‘dimer of dimers’ quaternary architecture.For clarity, only one of the two NR1/NR2 heterodimers is shown. The extracellular region of each subunit is made up of a tandem of bilobate ‘Venus-flytrap’ domains, the NTD and the ABD. In the extracellular region, the subunits dimerize at the level of the ABDs and probably also at the level of the NTDs. The NR2 ABD binds glutamate, whereas the NR1 ABD binds the co-agonist glycine (or D-serine).White arrows indicate binding sites for competitive agonists and antagonists. Thick orange arrows indicate sites known to bind allosteric modulators such as endogenous zinc (NR2A and NR2B NTDs) or ifenprodil-like compounds (NR2B NTDs), both acting as non-competitive antagonists. The ion-channel domain also forms binding sites for pore blockers such as endogenous Mg2+, MK-801, memantine or ketamine,acting as uncompetitive antagonists. Thin orange arrows indicate putative modulatory sites, which can bind either positive or negative allosteric modulators. The only known NMDAR antagonists that display strong subunit selectivity are the NR2 NTD ligands Zn2+, which selectively inhibits NR2A-containing receptors at nanomolar concentrations, and ifenprodil-like compounds, which selectively inhibit NR2Bcontaining receptors.
(Pierre Paoletti and Jacques Neyton (2007) NMDA receptor subunits: function and pharmacology. Curr. Opin. Pharmacol., 7, 39-47).