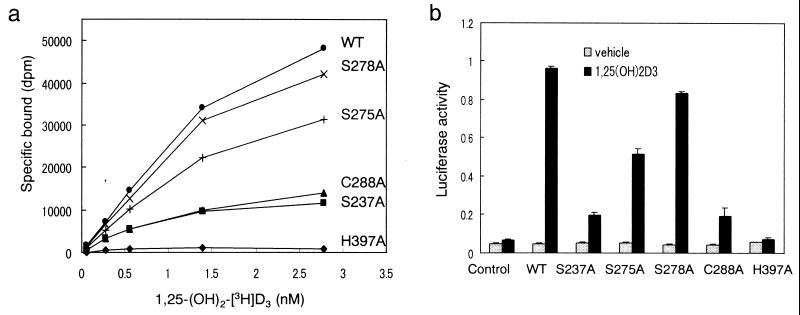
Figure 4.
Binding capability and transcriptional activity of the wild-type (WT) and mutant VDRs. (a) Specific binding to 1,25-(OH)2D3. The WT and the mutant hVDRs were synthesized in vitro in a rabbit reticulocyte lysate. The lysate was incubated with increasing concentrations of 1,25-(OH)2D3 for 16 h at 4°C. Bound and unbound ligands were separated by dextran-coated charcoal. (b) Transcriptional activity. COS-7 cells were cotransfected with WT or mutant hVDR expression vectors, SPPx3-TK-Luc as a reporter plasmid and pRL-CMV vector as an internal control. Before harvesting, cells were treated for 16 h with 10−8 M 1,25-(OH)2D3. Transactivation was determined by luciferase activity and normalized to the internal control.