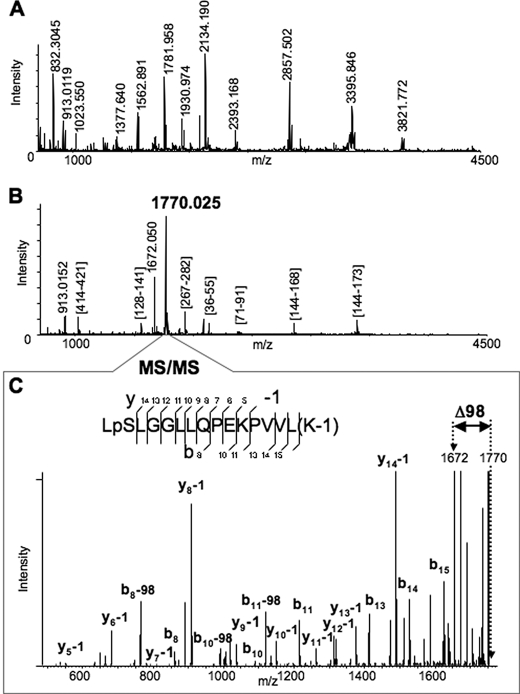
FIGURE 1.
Identification of the Ser478 phosphorylation site of CYP3A4 by MALDI-MSn analyses. A, MALDI-MS spectrum of Lys-C digested CYP3A4. Some of the enzymatic peptides are labeled in the spectrum. B, MALDI-MS spectrum of Lys-C digested CYP3A4 after the phosphopeptide enrichment performed as described in the text. All the detected peaks were subjected to MS/MS analysis to identify the peptides. The majority of the observed peaks was assigned to Lys-C enzymatic peptides of CYP3A4, except the two most abundant peaks, which were observed at m/z 1770.025 and 1672.050. C, MS/MS spectrum of the peptide at m/z 1770.025. The assignment of the observed fragments revealed the identity of the peptide (477–492) from CYP3A4 with two modifications. One modification is phosphorylation of Ser478 residue. Another is modification of Lys492 by 1 Da (see possible explanations in the text). The spectrum also revealed a prominent peak of loss of 98 Da from the parent peptide, which corresponds to the fragment with m/z 1672.050 also observed in the previous spectrum (B).