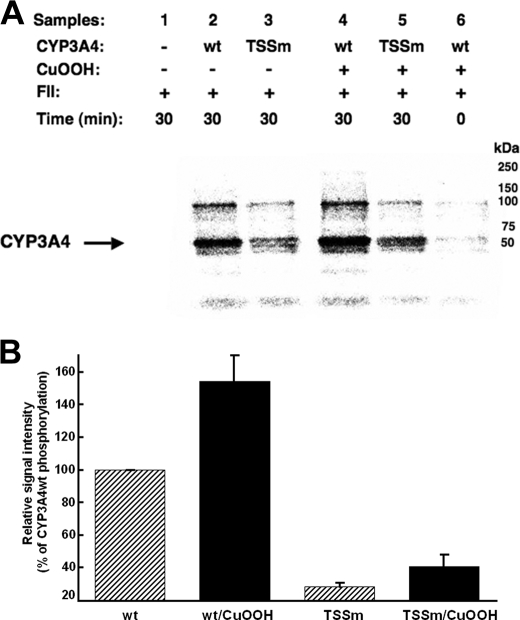
FIGURE 3.
In vitro phosphorylation of CYP3A4wt and its T264A/S420A/S478A mutant (TSSm) by FII in the presence of γ-S-[32P]ATP. A, relative extent of phosphorylation of each protein was examined by PhosphorImager scanning after Dynabead TALON extraction and SDS-PAGE analyses of comparably sized aliquots as detailed (see under “Experimental Procedures”). These data are representative of incubations carried out in at least three separate experiments. B, P450 phosphorylation (region between 50 and 250 kDa) was quantitated from the PhosphorImager scanned gels using ImageJ software. Values depicted represent the mean ± S.D. of three individual experiments. The phosphorylation of CuOOH-inactivated CYP3A4wt protein was increased statistically significantly over the corresponding native CYP3A4wt at p < 0.01. Similar statistically significant differences at p < 0.01 were also observed between the phosphorylation of native CYP3A4wt and that of the native TSSm protein, as well as between that of CuOOH-inactivated CYP3A4wt and CuOOH-inactivated TSSm protein. The difference between the phosphorylation of the native TSSm protein and its corresponding CuOOH-inactivated species was statistically significant at p < 0.05. wt, wild type.