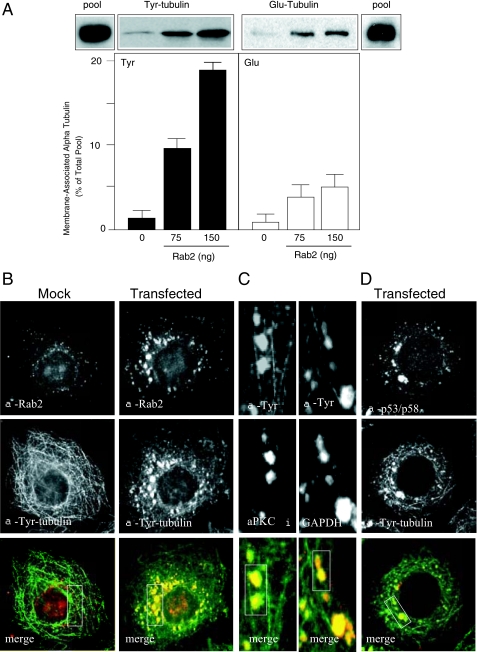
FIGURE 2.
α-Tubulin recruited to Rab2-treated membranes is predominantly tyrosinated. A, quantitative membrane binding assay was performed as described under “Experimental Procedures.” Salt-washed HeLa microsomes were incubated with cytosol and GTPγS in the presence or absence of purified Rab2 (75 or 150 ng) for 12 min at 32 °C. The membrane pellet was subjected to SDS-PAGE and transferred to nitrocellulose, and the blot was probed with anti-Tyr-tubulin monoclonal antibody and with anti-Glu-tubulin polyclonal antibody. The pool indicates total Tyr- and Glu-tubulin present in the cytosol added to each binding reaction. Results are presented as percent of total pool for each tubulin subtype. A representative Western blot from one of five independent experiments is shown. HeLa cells were mock-transfected or transfected with Rab2 cDNA. Thirty six hours post-transfection, cells were permeabilized, fixed, and then labeled with anti-Rab2 and anti-Tyr-tubulin (B) or with anti-Tyr-tubulin and anti-p53/p58 (D), as described under “Experimental Procedures.” C, high magnification of Rab2 cDNA-transfected cells labeled with anti-Tyr-tubulin and anti-aPKCι or anti-GAPDH. Boxed areas (yellow indicates co-localization) were analyzed using the Manders overlap co-localization, as described under “Experimental Procedures” and reported under “Results.” Rab2, GAPDH, aPKCι, and p53/p58 co-distribute with Tyr-tubulin-labeled VTCs.