Abstract
In adult rats, acute nicotine, the major psychoactive ingredient in tobacco smoke, stimulates the hypothalamic-pituitary-adrenal axis (HPA), resulting in activation of brain areas involved in stress and anxiety-linked behavior. However, in rat pups the first two postnatal weeks are characterized by hypo-responsiveness to stress, also called the 'stress non-responsive period' (SNRP). Therefore, we wanted to address the question if acute nicotine stimulates areas involved in the stress response during SNRP? To determine neuronal activation, the expression of the immediate-early genes c-fos and Arc was studied in the central nucleus of the amygdala (CeA), bed nucleus stria terminalis (BST) and paraventricular hypothalamic nucleus (PVN), which are areas involved in the neuroendocrine and central stress response. Rat pups received nicotine tartrate (2 mg/kg) or saline by i.p. injection at postnatal day (P) 5, 7 and 10 and their brains were removed after 30 min. We used semi-quantitative radioactive in situ hybridization with gene specific antisense cRNA probes in coronal sections. In control pups, c-fos expression was low in most brain regions, but robust Arc hybridization was found in several areas including cingulate cortex, hippocampus and caudate. Acute nicotine resulted in significant induction of c-fos expression in the PVN and CeA at P5, P7 and P10, and in the BST at P7 and P10. Acute nicotine significantly induced expression of Arc in CeA at P5, P7 and P10, and in the BST at P10. In conclusion, acute nicotine age dependently activated different brain areas of the HPA axis during the SNRP. After P7, the response was more pronounced and included the BST, suggesting differential maturation of the HPA axis in response to nicotine.
Keywords: anxiety, SNRP, nicotinic, amygdala, hypothalamus, development
INTRODUCTION
Stress-activated pathways include the neuroendocrine hypothalamic-pituitary-adrenal (HPA) axis and the central, limbic stress-loop (Avishai-Eliner et al., 2002). The HPA axis is the main mediator of the stress-response system which nicotine, the major psychoactive ingredient in tobacco smoke, can activate. This results in increased release of adrenocorticotropic hormone (ACTH) and glucocorticoids (CORT) in adult rats (Fuxe et al., 1989, Matta et al., 1998, Davis et al., 2005, Rhodes et al., 2001, Lutfy et al., 2006). Although nicotine can interact with peripheral neuronal nicotinic acetylcholine receptors (nAChR) located in the peripheral nervous system and in the adrenal medulla (Dávila-García et al., 2003), it is believed that nicotine acts centrally via activation of nAChRs located in the brainstem to stimulate the HPA axis. Lesions of brainstem catecholaminergic nuclei or administration of adrenergic receptor antagonists into brainstem catecholaminergic areas block nicotine induced release of ACTH (Matta et al., 1998). Therefore, the proposed mechanism involves a direct effect of nicotine on hindbrain noradrenergic nuclei, such as the nucleus tractus solitarii or locus coeruleus (LC), projecting to the hypothalamic paraventricular nucleus (PVN), resulting in nicotine stimulated norepinephrine release from noradrenergic nerve terminals. This in turn causes the release of corticotropin releasing factor and subsequently the secretion of ACTH from the pituitary, which then induces the release of CORT from the adrenal glands (Matta et al., 1998; Okada et al., 2003).
The central stress circuit involves the central nucleus of the amygdala (CeA) which projects to many regions involved in the stress-response, including the bed nucleus of the stria terminalis (BST), LC and PVN, suggesting a potential regulatory role for CeA in the neuroendocrine component of the stress response (Tsigos and Chrousos 2002, Gray et al., 1989; Herman and Cullinan 1997, Veening et al., 1984). Supporting this suggestion, electrical stimulation of the CeA has been shown to increase serum levels of both ACTH and CORT (Weidenfeld et al., 1997). The CeA also receives dense ascending noradrenergic projections from brainstem noradrenergic nuclei, including the LC, and from other lateral tegmental and medullary noradrenergic cell groups (Moore and Bloom, 1979, Cedarbaum and Aghajanian 1998, Cunningham and Sawchenko, 1988, Mason and Fibiger, 1979), and therefore could also be activated by nicotine.
The activation of brain areas involved in the stress-response via nAChR mediated mechanisms has been studied using the induction of the immediate early gene c-fos as a marker for neuronal activation (Herrera and Robertson, 1996). Recent studies have consistently shown that acute nicotine can activate central brain areas involved in the stress-response and increases c-fos mRNA expression in the CeA, PVN and the BST in adult and adolescent male rats (Salminen et al., 1999, Loughlin et al., 2006, Shram et al., 2007) confirming nicotine’s ability to stimulate stress-activated pathways.
However, during the first two weeks of postnatal life the HPA axis is characterized by a period of reduced responsiveness to stress (Vazquez et al., 2006). This period of adrenocortical quiescence has been termed the 'stress non-responsive period' (SNRP) (Sapolsky and Meaney, 1986). Thus, neonatal rat pups show very low basal CORT levels and an inability to respond to many stressors with increased CORT release (Levine 2005). Centrally, c-fos mRNA induction and corticotropin-releasing factor mRNA expression in the PVN are reduced in animals tested within the SHRP relative to post-SHRP animals suggesting attenuated stress-induced excitation of central neurons controlling release of ACTH (Dent et al., 2000a, Dent et al., 2000b).
Since the SNRP occurs during a critical period of brain development in which axonal outgrowth, synaptogenesis, and formation of key brain neurocircuits take place, dampening of HPA reactivity might be important for protecting the developing brain from glucocorticoid surges (Sapolsky and Meaney, 1986). However, the HPA axis can be activated during SNRP. Significant stressors applied during this period, such as maternal deprivation, can trigger a stress-response and result in life-long enhancement of both behavioral and neuroendocrine responsiveness to stress (Ladd et al., 2004, Levine, 1967, Levine, 2001). Thus, stressful events during this period strong enough to activate the stress-response modulate the development of stress circuitry and program future behavior (Francis et al., 1999).
Recent studies have shown that chronic exposure to nicotine during pre-or postnatal periods has long-lasting effects on anxiety-like behavior similar to prolong periods of maternal separation (Vaglenova et al., 2004, Huang et al., 2007a), suggesting that developmental nicotine exposure could trigger a stress-response during SNRP which could alter future anxiety behavior. However, the effects of nicotine during SNRP on brain areas involved in the central stress-response are not known. Therefore, we wanted to determine if an acute nicotine exposure could elicit activation of central brain areas associated with stress-activated pathways as seen in adults. In the present study we used semi-quantitative in situ hybridization to determine nicotine-induced gene expression of the immediate early genes c-fos and Arc (activity-regulated cytoskeletal associated protein, also known as Arg3.1) in the CeA, BST and PVN in postnatal rat pups.
EXPERIMENTAL PROCEDURES
Animals
Timed-pregnant Sprague-Dawley rat dams purchased from Harlan, Inc. (Houston, TX) arrived on gestation day 15–17, and were housed at the College of Medicine’s Animal Care Facility according to the rules of Texas A&M University Laboratory Animal Care Committee, and consistent with National Institute of Health guidelines. The first 24 hours after rat pups were born was considered as postnatal day (P) 0. On P5, P7 and P10 healthy pups of both sexes were randomly assigned to three different groups: non-handled control, saline injected control and nicotine injected groups (n=3). On the experimental day, pups were weighed and received an intra peritoneal (i.p.) injection with either saline (1 ml/kg 0.9% NaCl) or nicotine in saline (2 mg/kg nicotine di-(+)-tartrate, Sigma, St. Louis, MO, equivalent to 0.7 mg/kg nicotine). At the indicated age, pups of the saline and nicotine treatment groups were removed from the dam, placed in a small container and kept at 37°C. The pups received one injection each, which were spaced every five minutes between pups, alternating between saline and nicotine. Thirty minutes after receiving the injection, pups were decapitated, their brains quickly removed, frozen in −20°C isopentane and stored at −80°C until tissue preparation. Pups assigned to the non-handled control group remained with the dam until they were sacrificed.
Tissue preparation
Sections, 20 µm thick, were cut on a cryostat at −20°C and mounted onto poly-L-lysine coated glass slides. Sections were fixed with 4% paraformaldehyde for 1 hour at room temperature, followed by two 5-minute washes in 0.1 M phosphate buffer (PB), dried and stored with desiccant at −20°C until use.
c-RNA probe synthesis
The PGEM plasmid containing a 680 bp fragment of the c-fos gene was linearized and used as a template to synthesize c-fos cRNA probes in the sense and antisense orientations (cDNA kindly provided by Dr. Stanley J. Watson, University of Michigan, originally described by Dr. T. Curran, The Children’s Hospital of Philadelphia). A 3 kb transcript subcloned into pBSII(SK) (kindly provided by Dr. P.F. Worley, Johns Hopkins University) was used as a template to synthesize Arc sense and antisense probes. Probes were reversed transcribed in the presence of [35S]-UTP (MAXIscript™, Ambion, Austin, TX ), diluted in hybridization buffer at a concentration of 107 cpm/ml and stored at −20°C for no longer than 10 days.
A rat CRF template (578 bp) was kindly provided by Dr. Robert Thompson (University of Michigan, Ann Arbor, MI), and a Digoxigenin-labeled CRF antisense cRNA probe was synthesized using Dig-UTP (Roche Applied Science, Indianapolis, IN).
In situ hybridization
Tissue sections were processed according to a modification of a method previously described (Winzer-Serhan et al., 1999). Briefly, sections were incubated in proteinase K (0.05 mg/ml) for 10 min at room temperature, acetylated, and dehydrated through graded ethanol concentrations and air dried. Slide-mounted sections were incubated for 18 hours at 60°C with hybridization solution containing the 35S-labeled cRNA probes for c-fos or Arc in either the antisense or sense orientation. The next day sections were washed in 4× SSC, treated with RNase at 37°C, washed in SSC of decreasing salinity and followed by a hot wash in 0.1× SSC at 65°C. Slides were dehydrated through graded series of alcohols and dried. Sections were apposed to BioMax MR film (Kodak, Rochester, NY) together with 14C tissue standards (Amersham, Code RPA 504L, Batch 21, Buckinghamshire, UK) of known radioactivity. Following film development, sections were coated with liquid NTB emulsion (VWR International, West Chester, PA), developed after 3 weeks and counter stained with Cresyl-violet.
Double in situ hybridization
Sections were hybridized for 18 hours at 60°C with a 1:1 dilution of Dig-labeled antisense CRF cRNA probe (0.1 µg/ml) and 35S-labeled cfos antisense probe (2 × 107 cpm/ml) in hybridization buffer and processed for in situ hybridization as described above. After the hot wash, the slides were incubated in 100 mM Tris-HCl, 150 mM NaCl, pH 7.5 (GB1) for 5 min, followed by a 30 min incubation in 5% nonfat dry milk in GB1 plus 0.25% Triton-X. The alkaline-phosphatase conjugated anti-Dig Fab antibody (sheep) (Roche Applied Science, Indianapolis, IN), prepared as 1:1,000 dilution in GB1, was applied to the sections, and slides were incubated for 3 hours. The slides were washed three times for 1, 5, and 10 min in GB1. Slides were incubated with color reagent (200 µl of NBT/BCIP stock solution (18.75 mg/ml NBT, 9.4 mg/ml BCIP) in 10 ml of 100 mM Tris-HCl, 100 mM NaCl, 50 mM MgCl2, pH 9.50 (Roche Applied Science, Indianapolis, IN) at room temperature overnight. The slides were washed twice in 10 mM Tris-HCl, 1 mM EDTA, pH 8.0 and once in double deionized water, dehydrated with brief dips in graded ethanols (50, 70, 95, and 100%), air-dried and apposed to Kodak Biomax MR film for an appropriate period of time. After film development, slides were coated with 2% parlodion (SPI-Chem, West Chester, PA) in isoamylacetate, and dipped in liquid NTB emulsion (VWR, West Chester, PA). After 18 day exposure, slides were developed in Kodak developer D-19, fixed, cover-slipped, and analyzed.
Analysis and Quantification of results
The BST, PVN, and CeA were localized and analyzed using a rat brain atlas (Paxinos and Watson, 1998). Comparable film autoradiographic images derived with the antisense probes were quantitatively analyzed by using a computer-based image analysis system (Microcomputer Imaging Device, Imaging Research Inc., St. Catherine, Ontario, Canada, now InterFocus Imaging Ltd, Linton, UK). A calibration curve of radioactivity (nCi/g wet weight tissue) versus optical density was generated using [14C] standards. Regional values of optical density were converted to radioligand concentrations. Data were analyzed using two-way ANOVA using treatment and age as between subject factors by PC-based SPSS 12.0. Significance is defined as P≤ 0.05.
RESULTS
Nicotine induces c-fos mRNA in rat pups
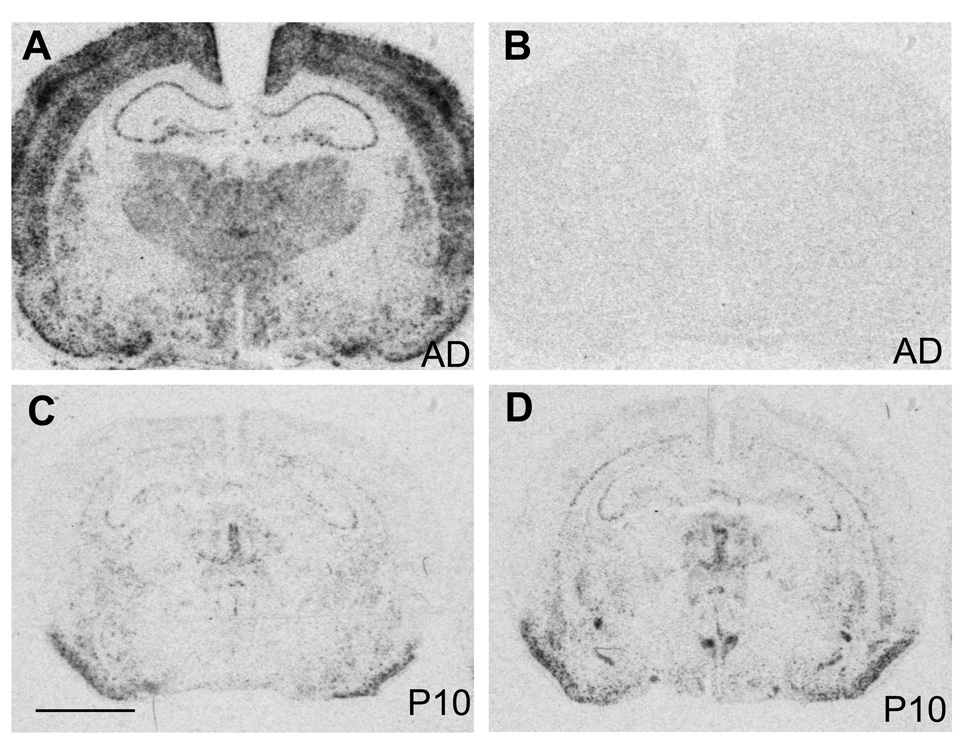
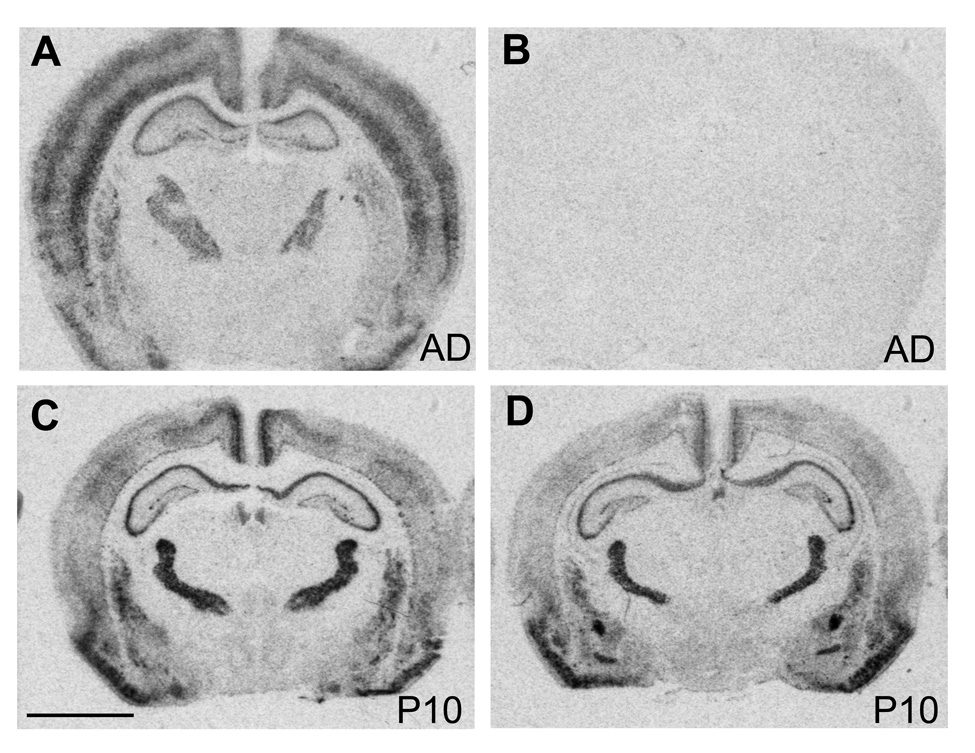
The specificity of the c-fos cRNA probe was tested in adult brain sections. In adult brains c-fos hybridization with the antisense probe was widely distributed at low to moderate levels throughout the brain with more intense signal in cortex (Fig. 1A), similar to previously published results (Nagahara and Handa, 1997). The sense probe resulted in only background levels of hybridization signal (Fig. 1B). The adult-like expression pattern was also detected in two week old rat pups (data not shown), but not in younger ones, where c-fos mRNA expression was very low throughout the brain (Fig. 1C, E). However, despite the low levels of c-fos expression in rat pups during the first and second postnatal week, nicotine induced robust c-fos mRNA expression in the areas of the central stress response including the BST, CeA and PVN, while other areas were mostly unaffected (Fig. 1D, F).
Figure 1.
Autoradiographic images of c-fos mRNA expression in forebrain sections from adult (A), and saline (C) and nicotine treated (D) postnatal day 10 rat brains. Non-specific hybridization was detected with a c-fos sense probe (B). Scale bar = 0.3 mm.
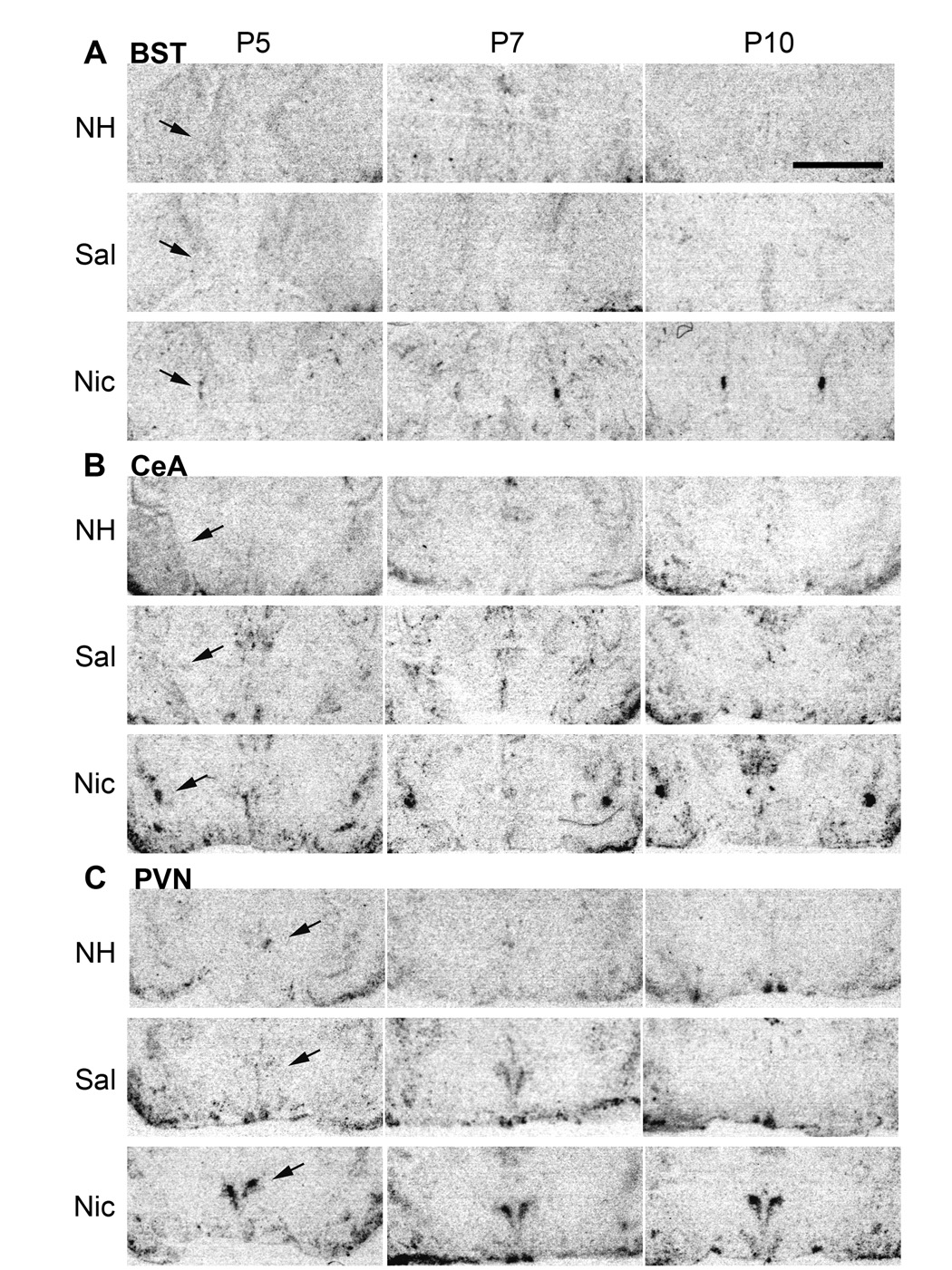
The ability of nicotine to induce c-fos mRNA expression in the BST, CeA and PVN was evaluated in rat pups ages P5, P7 and P10, in non-handled and saline injected controls, and nicotine injected animals (Fig. 2). In the BST, CeA and PVN, the expression of c-fos mRNA was low in saline and non-handled control postnatal rat pups (Fig. 2A, B, C).
Figure 2.
c-fos mRNA expression in brain sections from rat pups at postnatal day P5, P7, and P10, in non-handled (NH), saline (Sal) controls, and nicotine (Nic) animals, 30 minutes after either saline or nicotine (0.7 mg/kg) injection (control) in the bed nucleus stria terminalis (BST) (A), central nucleus of the amygdala (CeA) (B), and paraventricular nucleus of the hypothalamus (PVN) (C). Scale bar = 0.3 mm.
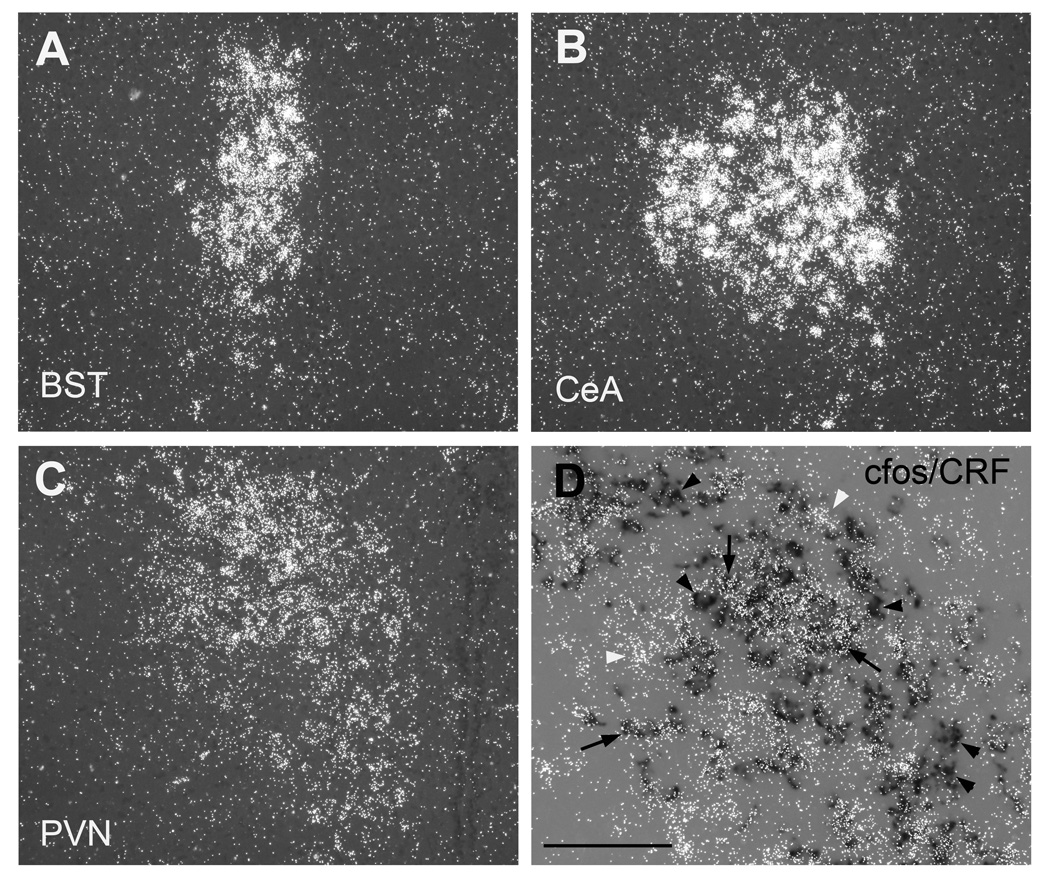
Darkfield microscopy was used to further analyze the anatomical location of nicotine-induced cfos expression (Fig. 3). In the BST, cfos expression was mainly localized in the lateral dorsal subdivision (Fig. 3A). In the CeA, nicotine-induced cfos expression was strongest in the capsular division, but robust signal was also detected in the lateral, and to a lesser extent in the medial regions (Fig. 3B). In the PVN of nicotine-treated animals, c-fos expressing cells were localized to both the medial parvocellular and the lateral magnocellular divisions, where double in situ hybridization showed that a large portion of CRF expressing cells exhibited nicotine-induced cfos mRNA expression (Fig. 3C, D). However, a few CRF positive neurons did not coexpress cfos, and some CRF negative neurons exhibited strong cfos mRNA expression.
Figure 3.
Acute nicotine-induced cfos mRNA expression in coronal brain sections from rat pups. Darkfield images of c-fos mRNA expression in (A) Bed nucleus of stria terminalis (BST), (B) Central nucleus of the amygdale (CeA), (C) hypothalamic paraventricular nucleus (PVN) after an acute treatment with nicotine at postnatal day ten. (D) colocalization of cfos mRNA (silver grains) and CRF (dark label) in the PVN after acute nicotine treatment at postnatal day seven. Scale bar = 200 µm in A – C, 100 µm in D.
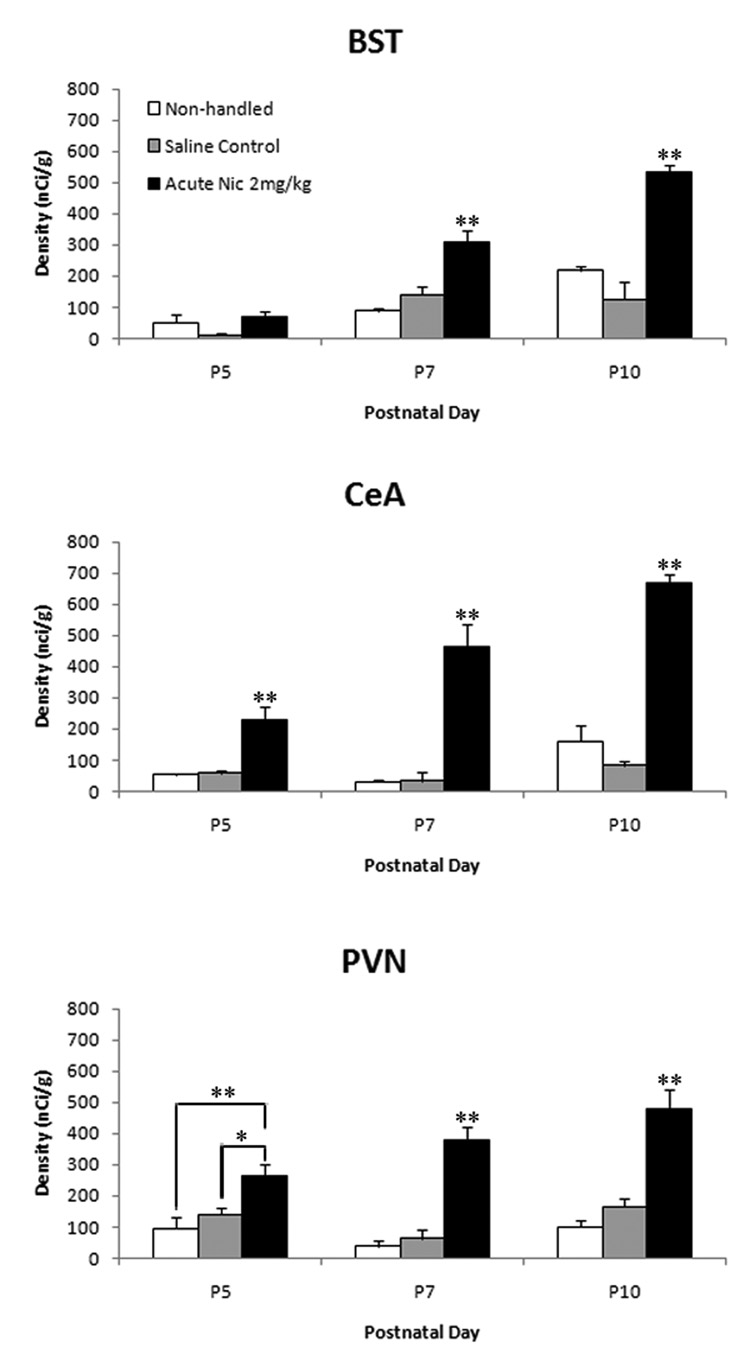
Quantification of the autoradiograms revealed that there was no significant difference in c-fos mRNA expression between non-handled and saline injected controls; therefore values of the both control groups were combined for the statistical analysis (Fig. 4). Nicotine induced c-fos mRNA expression in all three areas in an age dependent manner. Significant induction of c-fos mRNA was detected at P7 [p <0.001, F= 20.08] and P10 [p <0.001, F= 35.86] in the BST, and at P5, P7 and P10 in the CeA [p <0.001, F= 18.42, p <0.001, F= 31.18, p <0.001, F= 82.55, respectively] and PVN [p <0.05, F= 8.296, p <0.001, F= 35.2, p <0.001, F= 28.53, respectively] (Fig. 4). The response to nicotine increased with age and the most robust response was detected at P10 in all three areas [BST: p <0.001, F= 18.25, CeA: p <0.001, F= 18.88, PVN: p =0.004, F= 7.4].
Figure 4.
Quantitative analysis of c-fos mRNA expression in the bed nucleus stria terminalis (BST), central nucleus of the amygdala (CeA) and paraventricular nucleus of the hypothalamus (PVN) in non-handled, saline injected and nicotine injected rat pups at P5, P7, and P10. Values are expressed as mean ± SEM; n = 3. *P < 0.01 **P < 0.001.
Nicotine induces Arc mRNA in early postnatal rat pups
The specificity of the Arc cRNA probe was tested in adult brain sections. Arc hybridization with the antisense probe was strong in cortex and hippocampus and moderate in other regions such as the caudate putamen and reticular nucleus of the thalamus, whereas other thalamic nuclei and the hypothalamus exhibited no hybridization signal (Fig. 5A) similar to previously published results (Lyford et al., 1995). The sense probe resulted in only background levels of hybridization signal (Fig. 5B). In contrast to the low c-fos expression, in rat pups Arc mRNA expression was strong and widespread in several forebrain areas including cortex, caudate and hippocampus, exhibiting a similar expression pattern as detected in adults (Fig. 5C, E). Nicotine selectively induced robust Arc mRNA expression in the CeA and the BST in postnatal animals, but other areas including the hypothalamic PVN were unaffected (Fig. 5D, F).
Figure 5.
Autoradiographic images of Arc mRNA expression in forebrain sections from adult (A), and saline (C) or nicotine (D) treated postnatal day 10 rat brains. Non-specific hybridization was detected with an Arc sense probe (B). Scale bar = 0.3 mm.
Detailed analysis revealed that in the BST and CeA, Arc mRNA expression was low in saline and non-handled control pups and there was no significant difference between the two control groups (Fig. 6A, B). Nicotine specifically induced Arc expression in the BST and CeA but not in the PVN in rat pups. Darkfield analysis showed that nicotine-stimulated Arc expression was mainly located in the lateral dorsal part of the BST (Fig. 7A), and in the capsular and lateral divisions of the CeA (Fig. 7B). This spatial distribution was similar to that of nicotine-induced cfos expression. However, coexpression studies would be needed to verify that both IEG are activated by nicotine in the same neurons.
Figure 6.
Arc mRNA expression in brain sections from rat pups at postnatal day P5, P7, and P10, in non-handled (NH), saline (Sal) controls, and nicotine (Nic) animals, 30 minutes after either saline or nicotine (0.7 mg/kg) injection (control) in the bed nucleus stria terminalis (BST) (A), and central nucleus of the amygdala (CeA) (B). Scale bar = 0.3 mm.
Figure 7.
Acute nicotine-induced Arc mRNA expression in coronal brain sections from rat pups. Darkfield images of Arc mRNA expression in (A) Bed nucleus of stria terminalis (BST), (B) Central nucleus of the amygdale (CeA) after an acute treatment with nicotine at postnatal day ten. Scale bar = 200 µm.
Quantification of the autoradiograms revealed that there was significant induction of Arc mRNA detected at P7 [p <0.05, F= 6.37] and P10 [p <0.001, F= 130.76] in the BST and in the CeA at P5 [p <0.05, F= 7.91], P7 [p <0.001, F= 19.00] and P10 [p <0.001, F= 270.62] (Fig. 8). The response to nicotine was more pronounced with increasing age and the most robust response was detected at P10 in both regions [BST: p <0.001, F= 56.76, CeA: p <0.001, F= 16.00].
Figure 8.
Quantitative analysis of Arc mRNA expression in the bed nucleus stria terminalis (BST) and central nucleus of the amygdala (CeA) in non-handled, saline injected and nicotine injected rat pups at P5, P7, and P10. Values are expressed as mean ± SEM; n = 3. *P < 0.01 **P < 0.001.
DISUCCION
This is the first study to demonstrate that acute nicotine exposure can induce c-fos mRNA expression in brain centers associated with anxiety in rat pups as young as 5 days old. In addition, the activation of Arc mRNA indicates increased synaptic activity in the BST and CeA which could lead to altered connectivity and contribute to the long-term behavioral changes seen after chronic neonatal nicotine exposure (Huang et al., 2007a).
Expression of cfos and Arc in neonates
In control postnatal rat pups ages P5, P7 and P10 c-fos mRNA expression was very low, and restricted to a few frontal brain areas such as piriform cortex (note: midbrain and hindbrain areas were not evaluated). Expression patterns between non-handled and saline injected controls were identical during the first and second postnatal week indicating that a saline injection or handling was not sufficient to stimulate c-fos expression in neonates. In contrast, in adults and similarly in older pups (P15, data not shown) the expression was strong and widespread, especially in neocortex. Since c-fos expression is considered a marker for brain activity, this suggests that during the first two postnatal weeks frontal brain areas are quiet and not easily activated in response to stimuli.
Arc mRNA expression on the other hand, was widespread in control pups of all ages including P5, and the expression pattern was similar to those in adults with strong expression in cortical areas such as neocortex and hippocampus, and no expression in thalamus and hypothalamus, except for the reticular thalamic nucleus. There was no evidence for differential expression between non-handled and saline injected controls indicating that an injection or handling was not sufficient to stimulate altered Arc expression in young pups. The discrepancy between the low level c-fos expression and the moderate to strong Arc expression in neonates was surprising because both genes are associated with neuronal activity (Morgan and Curran, 1991, Vazdarjanova et al., 2002). In adults, the role of Arc as a marker of dendritic and synaptic activity in response to stimulation has been well established (Steward and Worley 2001, Pei et al 2003, Bramham 2007). Arc’s function during development is less well understood. However, it has been suggested that Arc is involved in synaptogenesis by modifying dendritic architecture associated with cytoskeletal proteins (Fujimoto et al 2004, Wang and Pickel 2004), and thus could be critically involved in circuit formation during development.
Nicotine-induced expression of cfos and Arc
Despite the low levels of c-fos expression in control newborns, an acute injection of nicotine strongly induced c-fos mRNA expression in the BST, CeA and PVN, and in a few other forebrain areas such as the cortical subplate and medial habenula. However, an acute injection of nicotine did not result in widespread c-fos expression or activated areas which display expression in older control animals such as more superficial cortical layers. Thus, nicotine-induced c-fos mRNA expression marks specifically a few restricted areas, mostly related to the central stress-response pathway in postnatal rat pups. Nicotine-induced c-fos expression in the PVN, BST and CeA has also been reported in adolescent and adult rats (Cao et al., 2007, Loughlin et al., 2006, Mathieu-Kia et al., 1998, Matta et al., 1993, Park et al., 2006, Salminen et al., 1999, Shram et al., 2007), and therefore, seems to reflect an age independed response to nicotine. Induction of c-fos is often used to evaluate stress-related responses because it can be triggered by a variety of stressors (Chan et al., 1993, Reyes et al., 2003, Hsu et al., 2007). Thus, nicotine seems to activate the central stress response system in postnatal, adolescent and adult animals.
It was however surprising that nicotine could activate the stress-related areas in newborn rat pups, because the first two weeks of postnatal life are characterized by relative hyporesponsiveness to stressful stimuli (Sapolsky and Meaney, 1986, Vazquez et al., 2006). It is not known if nicotine stimulated a pituitary-adrenal response in 5 to 10 day old pups, an age with only minimal stress-induced CORT release (Galeeva et al., 2006). However, the central component of the stress-response pathway can be activated by stressful stimuli during the SHRP (Smith et al., 1997, Dent et al., 2000). In particular strong stressors are capable of activating a stress-response during this period. Therefore, the results suggest that nicotine acts like a strong stressor in neonates. Increased Arc expression in response to nicotine has been reported in forebrain regions of adolescent rats (Schochet et al., 2005) but this is the first study to show that nicotine can rapidly induce Arc mRNA expression in areas associated with the central stress response. Similar to the pattern detected for cfos, nicotine-stimulated Arc mRNA expression was found in the CeA and BST of newborn rats. The induction of Arc mRNA has been associated with glutamate driven excitatory synapse formation at postsynaptic sites (Steward and Worley, 2001), and with LTP and memory consolidation (Guzowski et al., 2000, Plath et al., 2006), and might reflect alterations in long-term memory formation and postsynaptic signaling efficacy (Shepherd et al., 2006, Guzowski et al., 2000). Nicotine, via activation of nAChRs, facilitates glutamatergic transmission (Barazangi and Role, 2001, Giocomo and Hasselmo, 2007). Thus, nicotine might increase neuronal activity, as indicated by c-fos expression, and increase excitatory transmission, as indicated by Arc expression, along the central stress pathway. The increased activity could facilitate synapse formation, resulting in strengthened connectivity of ascending brainstem noradrenergic projections to the CeA, and projections from the CeA to other regions including the BST, which then result in long-term behavioral changes in response to stress. The findings from this study could point to a possible underlying mechanism for the long-term behavioral effects on anxiety seen after chronic developmental nicotine exposure described in recent studies (Vaglenova et al., 2004, Huang et al., 2007a, Slawecki et al., 2003).
However, nicotine also stimulates the release of GABA which is the primary inhibitory neurotransmitter in adults (Guo et al., 1998, Maggi et al, 2001). During development GABA transmission is often established before glutamatergic transmission, and is often excitatory in immature neurons as a result of high intracellular chloride concentrations (Owens et al., 1999). The shift in neuronal electrophysiological phenotype usually occurs during the first postnatal weeks (Owens et al., 1999, Ben-Ari et al., 2007). Thus, it is possible, that nicotine stimulates GABA release, and induction of cfos and Arc is mediated by excitatory postsynaptic GABA transmission in the developing brain. However, nicotine’s effect on the CeA, BST and PVN increased with age, and a similar response was detected in adolescent and adult rats, suggesting that nicotine induction of cfos and Arc is mediated by mature neuronal mechanism.
Possible nAChRs involved in nicotine induced cfos and Arc expression
At this point the type of nAChR(s) involved in nicotine’s regulation of cfos and Arc expression remains unknown, and further studies are needed to address this question. Homomeric and heteromeric nAChRs are widely expressed in the developing brain including the amygdale and hypothalamus (Tribollet et al., 2004). Therefore, nicotine could directly activate postsynaptic nAChRs located in the CeA, BST and PVN. However, except for the prominent expression of α7 nAChR subunit mRNA in the PVN, there was no clear correlation between nAChR binding sites and induction of either cfos or Arc by nicotine, and there appeared to be no correlation between nAChR subunit expression in the BST, CeA or PVN (Huang et al., 2007b, Winzer-Serhan and Leslie, 1997, unpublished results) and induction of cfos or Arc. In general, brain areas with high numbers of heteromeric or homomeric nAChRs binding sites during postnatal development, such as the thalamus or the hippocampus, respectively (Huang et al., 2007b), exhibited little or no induction by nicotine. The only noticeable exceptions were the cortical subplate, where high numbers of heteromeric nAChRs are found and nicotine induced cfos expression, and α7 expression in the PVN. However, using an α7 specific agonist (PNU) did not activate cfos expression in the PVN, or any other area studied suggesting that nicotine did not activate cfos expression in the PVN via postsynaptic activation of α7 receptors in the PVN (unpublished results).
Alternatively, as previous studies have suggested, nicotine could act indirectly, via activation of nAChRs expressed on brainstem catecholamine nuclei stimulating transmitter release from presynaptic terminals in the amygdala and hypothalamus, which then results in the release of CRF from neurons located in the PVN (Matta et al., 1998, Okada et al., 2003). Our results show nicotine-induced cfos expression in CRF positive neurons similar to the findings by Loughlin et al. (2006), and in support of an indirect activation mechanism.
Conclusion
Acute nicotine activates brain areas associated with the central stress response system in neonatal rat pups during a developmental period of hyporesponsiveness to stress. This indicates that developmental nicotine can act like a strong stressor in neonates and could permanently alter the response to stress.
Acknowledgement
This study was supported by NIH grant #DA016487.
List of abbreviations
- ACTH
adrenocorticotropic hormone
- Arc
activity-regulated cytoskeletal associated protein
- BST
bed nucleus stria terminalis
- CeA
central nucleus of the amygdala
- CRF
Corticotrophin releasing factor
- HPA
hypothalamic-pituitary-adrenal axis
- LC
locus coeruleus
- nAChR
neuronal nicotinic acetylcholine receptors
- PVN
paraventricular hypothalamic nucleus
- SNRP
stress non-responsive period
- CORT
glucocorticoids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazangi N, Role LW. Nicotine-induced enhancement of glutamatergic and GABAergic synaptic transmission in the mouse amygdala. J Neurophysiol. 2001;86:463–474. doi: 10.1152/jn.2001.86.1.463. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Bramham CR. Control of synaptic consolidation in the dentate gyrus: mechanisms, functions, and therapeutic implications. Prog Brain Res. 2007;163:453–471. doi: 10.1016/S0079-6123(07)63025-8. [DOI] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Keyler DE, Pentel PR, Leslie FM. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology. 2007;32:2025–2035. doi: 10.1038/sj.npp.1301327. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978;178:1–16. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- Chan RK, Brown ER, Ericsson A, Kovács KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Dávila-García MI, Musachio JL, Kellar KJ. Chronic nicotine administration does not increase nicotinic receptors labeled by [125I]epibatidine in adrenal gland, superior cervical ganglia, pineal or retina. J Neurochem. 2003;85:1237–1246. doi: 10.1046/j.1471-4159.2003.01774.x. [DOI] [PubMed] [Google Scholar]
- Davis KW, Cepeda-Benito A, Harraid JH, Wellman PJ. Plasma corticosterone in the rat in response to nicotine and saline injections in a context previously paired or unpaired with nicotine. Psychopharmacology. 2005;180:466–472. doi: 10.1007/s00213-005-2185-7. [DOI] [PubMed] [Google Scholar]
- Dent GW, Okimoto DK, Smith MA, Levine S. Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology. 2000a;71:333–342. doi: 10.1159/000054554. [DOI] [PubMed] [Google Scholar]
- Dent GW, Smith MA, Levine S. Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology. 2000b;141:1593–1598. doi: 10.1210/endo.141.5.7455. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor--norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Andersson K, Eneroth P, Härfstrand A, Agnati LF. Neuroendocrine actions of nicotine and of exposure to cigarette smoke: medical implications. Psychoneuroendocrinology. 1989;14:19–41. doi: 10.1016/0306-4530(89)90054-1. [DOI] [PubMed] [Google Scholar]
- Galeeva A, Ordyan N, Pivina S, Pelto-Huikko M. Expression of glucocorticoid receptors in the hippocampal region of the rat brain during postnatal development. J Chem Neuroanat. 2006;31:216–225. doi: 10.1016/j.jchemneu.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. Epub 2007 Jul 20. [DOI] [PubMed] [Google Scholar]
- Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- Guo JZ, Tredway TL, Chiappinelli VA. Glutamate and GABA release are enhanced by different subtypes of presynaptic nicotinic receptors in the lateral geniculate nucleus. J Neurosci. 1998;18:1963–1969. doi: 10.1523/JNEUROSCI.18-06-01963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamopituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Hsu HR, Chen TY, Chan MH, Chen HH. Acute effects of nicotine on restraint stress-induced anxiety-like behavior, c-Fos expression, and corticosterone release in mice. Eur J Pharmacol. 2007;566:124–131. doi: 10.1016/j.ejphar.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Liu X, Griffith WH, Winzer-Serhan UH. Chronic neonatal nicotine increases anxiety but does not impair cognition in adult rats. Behav Neurosci. 2007a;121:1342–1352. doi: 10.1037/0735-7044.121.6.1342. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Abbott LC, Winzer-Serhan UH. Effects of chronic neonatal nicotine exposure on nicotinic acetylcholine receptor binding, cell death and morphology in hippocampus and cerebellum. Neuroscience. 2007b;146:1854–1868. doi: 10.1016/j.neuroscience.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo–pituitary–adrenal axis following neonatal maternal separation. Biol. Psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Islas MI, Cheng MY, Lee AG, Villegier AS, Leslie FM. Nicotine modulation of stress-related peptide neurons. J Comp Neurol. 2006;497:575–588. doi: 10.1002/cne.20999. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Brown MC, Nerio N, Aimiuwu O, Tran B, Anghel A, Friedman TC. Repeated stress alters the ability of nicotine to activate the hypothalamic-pituitary-adrenal axis. J Neurochem. 2006;99:1321–1327. doi: 10.1111/j.1471-4159.2006.04217.x. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536(Pt 1):89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason ST, Fibiger HC. Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979;187:703–724. doi: 10.1002/cne.901870405. [DOI] [PubMed] [Google Scholar]
- Mathieu-Kia AM, Pages C, Besson MJ. Inducibility of c-Fos protein in visuo-motor system and limbic structures after acute and repeated administration of nicotine in the rat. Synapse. 1998;29:343–354. doi: 10.1002/(sici)1098-2396(199808)29:4<343::aid-syn6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Matta SG, Foster CA, Sharp BM. Nicotine stimulates the expression of cFos protein in the parvocellular paraventricular nucleus and brainstem catecholaminergic regions. Endocrinology. 1993;132:2149–2156. doi: 10.1210/endo.132.5.8386611. [DOI] [PubMed] [Google Scholar]
- Matta SG, Fu Y, Valentine JD, Sharp BM. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinology. 1998;23:103–113. doi: 10.1016/s0306-4530(97)00079-6. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Handa RJ. Age-related changes in c-fos mRNA induction after open-field exposure in the rat brain. Neurobiol Aging. 1997;18:45–55. doi: 10.1016/s0197-4580(96)00166-2. [DOI] [PubMed] [Google Scholar]
- Okada S, Shimizu T, Yokotani K. Extrahypothalamic corticotropin-releasing hormone mediates (−)-nicotine-induced elevation of plasma corticosterone in rats. Eur J Pharmacol. 2003;473:217–223. doi: 10.1016/s0014-2999(03)01966-6. [DOI] [PubMed] [Google Scholar]
- Owens DF, Liu X, Kriegstein AR. Changing properties of GABA(A) receptor-mediated signaling during early neocortical development. J Neurophysiol. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- Park MK, Loughlin SE, Leslie FM. Gestational nicotine-induced changes in adolescent neuronal activity. Brain Res. 2006;1094:119–126. doi: 10.1016/j.brainres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd edition. San Diego: Academic Press; 1998. [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bösl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, O'Toole SM, Czambel RK, Rubin RT. Male-female differences in rat hypothalamic-pituitary-adrenal axis responses to nicotine stimulation. Brain Res Bull. 2001;54:681–688. doi: 10.1016/s0361-9230(01)00488-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Salminen O, Seppä T, Gäddnäs H, Ahtee L. The effects of acute nicotine on the metabolism of dopamine and the expression of Fos protein in striatal and limbic brain areas of rats during chronic nicotine infusion and its withdrawal. J Neurosci. 1999;19:8145–8151. doi: 10.1523/JNEUROSCI.19-18-08145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochet TL, Kelley AE, Landry CF. Differential expression of arc mRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neuroscience. 2005;135:285–297. doi: 10.1016/j.neuroscience.2005.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Acute nicotine enhances c-fos mRNA expression differentially in reward-related substrates of adolescent and adult rat brain. Neurosci Lett. 2007;418:286–291. doi: 10.1016/j.neulet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacol Biochem Behav. 2003;75:355–361. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Smith MA, Kim SY, van Oers HJ, Levine S. Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology. 1997;138:4622–4628. doi: 10.1210/endo.138.11.5529. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150:159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, López JF, Levine S. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121:83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Activity-regulated cytoskeleton-associated protein arc is targeted to dendrites and coexpressed with mu-opioid receptors in postnatal rat caudate-putamen nucleus. J Neurosci Res. 2004;77:323–333. doi: 10.1002/jnr.20173. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Itzik A, Feldman S. Effect of glucocorticoids on the adrenocortical axis responses to electrical stimulation of the amygdala and the ventral noradrenergic bundle. Brain Res. 1997;754:187–194. doi: 10.1016/s0006-8993(97)00078-4. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Broide RS, Chen Y, Leslie FM. Highly sensitive radioactive in situ hybridization using full length hydrolyzed riboprobes to detect alpha 2 adrenoceptor subtype mRNAs in adult and developing rat brain. Brain Res Brain Res Protoc. 1999;3:229–241. doi: 10.1016/s1385-299x(98)00043-9. [DOI] [PubMed] [Google Scholar]