Figure 5. Comparison of the iron-histidine bond geometry.
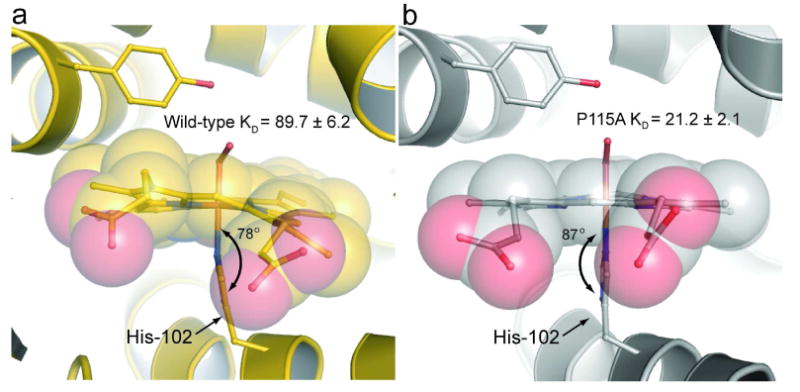
Shown are the iron-histidine tilts of a) wild-type Tt H-NOX (gold) and b) P115A (silver). The least squares plane of the heme was calculated using the 4 pyrrole nitrogens of the heme (other atoms were excluded due to the high degree of distortion) and the five imidazole ring atoms of H102 using MOLEMAN2 (22). Wild-type has a tilt of 78° whereas P115A has a tilt of 87°.
