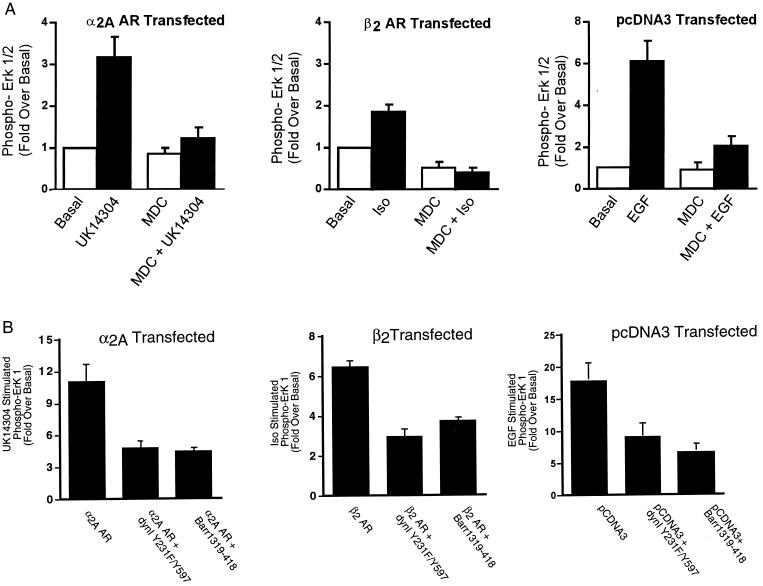
Figure 2.
The effect of chemical and transfectable inhibitors of clathrin-mediated endocytosis on α2A AR- and β2 AR-mediated ERK 1/2 phosphorylation. (A) Cells transiently expressing the α2A AR-, the β2 AR-, or vector-transfected cells were pretreated with 300 μM MDC before a 5-minute stimulation with 1 μM UK14304 (Left), 1 μM isoproterenol (Center), or 10 ng/ml EGF (Right). Aliquots of whole-cell lysate (approximately 30 μg of protein per lane) were resolved by SDS/PAGE, and ERK 1/2 phosphorylation was detected by protein immunoblotting by using rabbit polyclonal phospho-MAP kinase-specific IgG. Data are expressed as the fold ERK 1/2 phosphorylation over the basal value in appropriately transfected cells. The data shown are the mean ± SEM of four independent experiments. (B) Cells in 100-mm dishes were transiently transfected with a HA-tagged ERK-1 plasmid (0.5 μg) together with the α2A AR (2 μg, Left), the β2 AR (2 μg, Center), or pCDNA3 (Right) alone or with either dynamin I Y231F/Y597F (7.5 μg) or β-arrestin 1 318–419 (7.5 μg). One day after transfection, cells were split into two 100-mm dishes and serum-starved overnight. After stimulation for 5 minutes with either 100 nM UK14304 (α2A AR), 1 μM isoproterenol (β2 AR), or 1 ng/ml EGF (EGFR), cell lysates were prepared, and the HA–ERK-1 was immunoprecipitated. Immunoblots were probed with both an anti-phospho-ERK 1/2 and a total ERK 1/2 antibody. Under each condition, data are expressed as the fold ERK 1/2 phosphorylation over the unstimulated. Data shown are the mean ± SEM of three independent experiments.