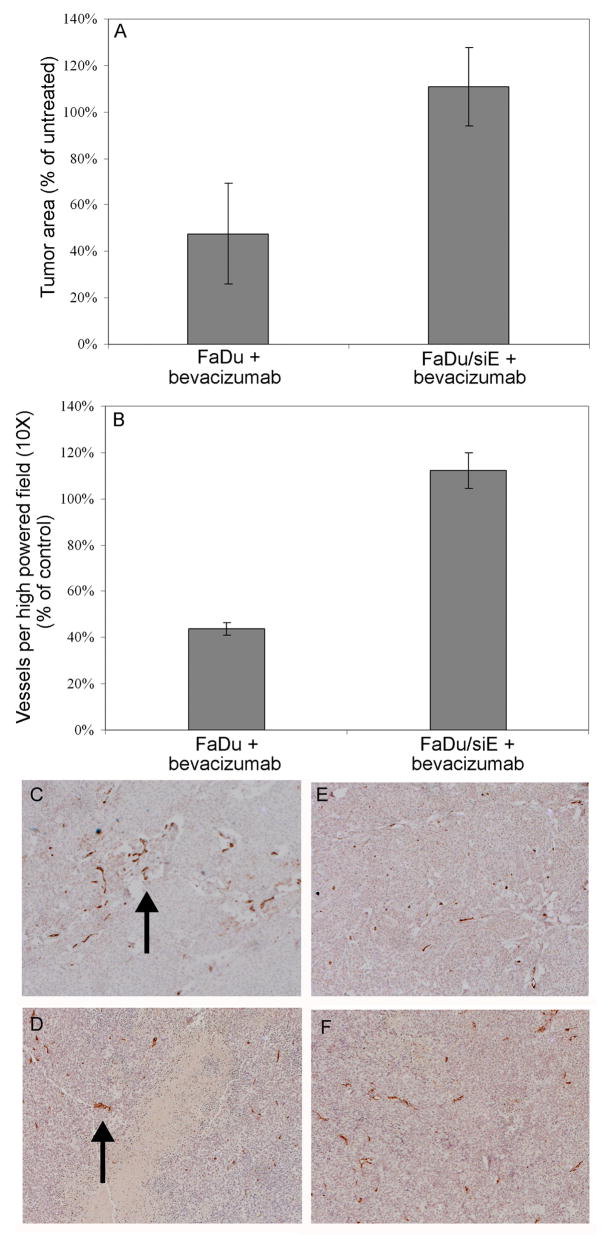
Fig. 3.
EMMPRIN expression required for bevacizumab response in vivo. (A) bevacizumab was effective in treating HNSCC xenografts in EMMPRIN expressing FaDu tumors (P = 0.0013), but response was not seen in tumors with knockdown EMMPRIN expression (FaDu/siE, P = 0.7942). Analysis of xenograft samples by immunohistochemistry staining for CD31 (B) revealed that bevacizumab decreased microvascular density of tumors that expressed EMMPRIN (P = 0.005), but had no effect on the vascularity of FaDu/siE tumors (P = 0.48). Immunohistochemistry of CD31 expression by FaDu control tumors (C) and tumors treated with bevacizumab (D) compared to FaDu/siE control (E) and treated (F) tumors. Data are normalized as percentage of untreated controls. Arrows indicate vascularity.