Figure 3. PrxI backside interaction with Srx.
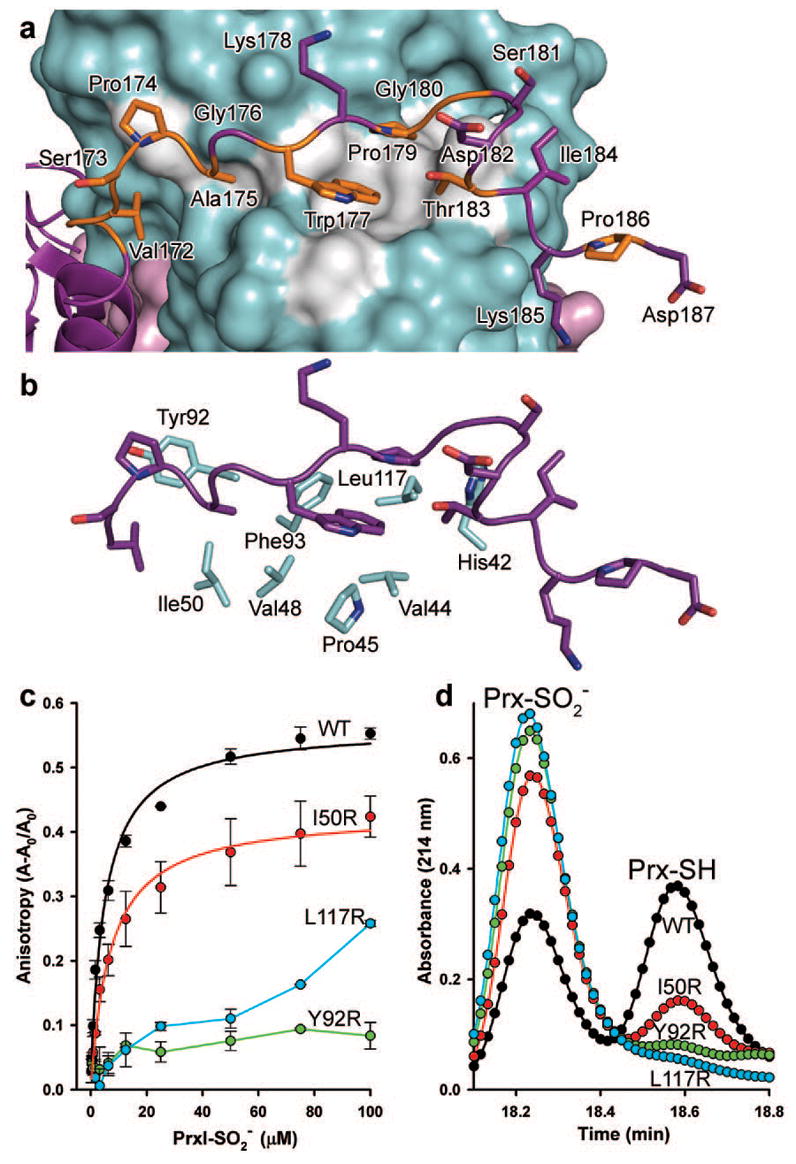
a, The C-terminal tail of PrxI binds to Srx. Conserved residues of PrxI are coloured orange, and the conserved surface of Srx is coloured grey. b, Surface residues of Srx that interact with the C terminus of PrxI. c, interactions measured in solution by changes in fluorescence anisotropy with Oregon Green 514-labelled Srx variants. Data obtained from representative, duplicate titrations are expressed as the fractional change in anisotropy, (A–A0)/A0, versus the concentration of decameric added, with the error bars indicating s.d. The data for WT Srx and the I50R mutant were fitted to a single-site, saturable binding model. d, Representative HPLC traces from the activity analysis of Srx variants.
