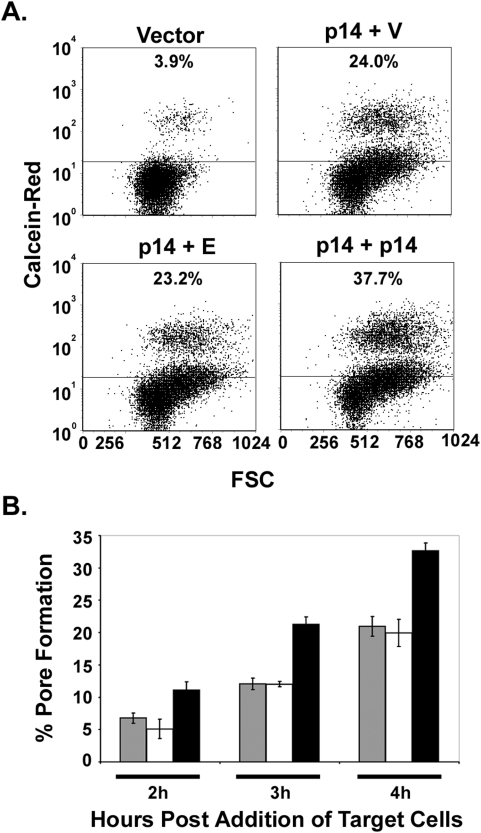
Figure 3. The p14 endodomain functions downstream of stable pore formation.
(A) QM5 cells were co-transfected to express p14, EGFP, and either empty vector (p14+V), the p14 endodomain (p14+E), or full-length p14 (p14+p14), and 3 h post-transfection were over-seeded with Vero cells labelled with calcein red AM. The cells were co-cultured for 4 h to allow cell–cell fusion to proceed, then trypsinized, and single-cell suspensions were analyzed by flow cytometry. EGFP-expressing donor cells were gated, and the percent donor cells that acquired calcein red were quantified and plotted versus the forward scatter (FSC). Donor cells transfected with empty vector instead of p14 (Vector) served as a control for fusion-independent dye transfer. Data is representative of two experiments conducted in triplicate. (B) A time course analysis of the experiment described in (A). The percent donor cells positive for calcein red, minus the background from vector-transfected donor cells, is graphed as percent pore formation. Results are the mean±S.D. from a representative experiment in triplicate. Cells were transfected with p14+vector (grey), p14+endodomain (white), or a double-dose of p14 (black).