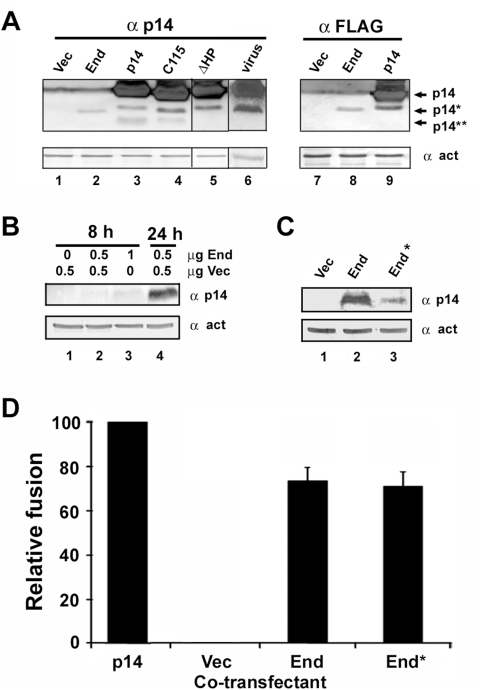
Figure 4. Endogenous in vivo generation of the p14 endodomain.
(A) Cell lysates from QM5 cells infected with reptilian reovirus (lane 6), or transfected with empty vector (lanes 1 and 7) or with plasmids expressing the p14 endodomain (End, lane 2), full length p14 (p14, lane 3), a C-terminal 10-residue (C115, lane 4) or N-terminal 21-residue (ΔHP, lane 5) truncation of p14, or N- (lane 8) or C- (lane 9) terminally FLAG-tagged versions of the endodomain or p14 were processed for Western blotting at 20 h post-infection or 12 h post-transfection using antibodies against p14, the FLAG epitope, or β-actin. The migration of full-length p14, the p14 endodomain (p14*), and the presumed p14 ectodomain (p14**) fragments are indicated on the right. Lane 5 was spliced in from the same blot as lanes 1–4; lane 6 was spliced in from a separate blot. (B) QM5 cells were transfected with the indicated amounts of empty vector (Vec) or with the p14 endodomain expression plasmid (End). At 8 or 24 h post-transfection, cell lysates were harvested and processed for Western blotting using antibodies against p14 or β-actin. (C) QM5 cells were transfected with the p14 endodomain expression plasmid (End) or with a plasmid expressing p14 from a sub-optimal translation start codon (End*). Cell lysates were harvested at 24 h post-transfection and processed for Western blotting using antibodies against p14 or β-actin. (D) Cells transfected with 0.5 µg of the p14 expression plasmid were co-transfected with 0.5 µg of plasmids expressing authentic p14 (p14), empty vector (Vec), the p14 endodomain (End), or expressing p14 from a sub-optimal translation start codon (End*). The extent of cell–cell fusion in triplicate samples was quantified as described in Figure 1A, and results are presented as the relative level of syncytium formation±S.D. from a single experiment, setting the cells co-transfected with authentic p14 as 100% fusion enhancement and those co-transfected with empty vector as 0% fusion enhancement.