Figure 2.
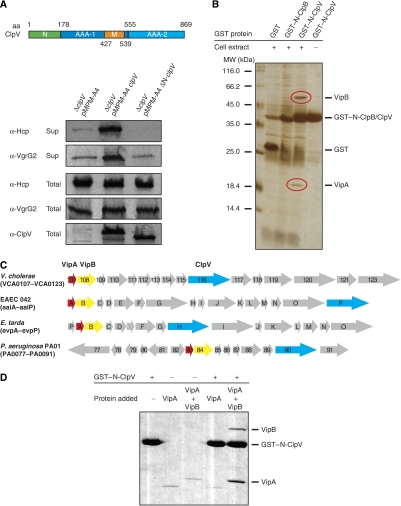
The ClpV N-domain is essential for ClpV activity and mediates binding to the VipA/VipB complex. (A) Upper part: schematic domain organization of V. cholerae ClpV. N: N-terminal domain; M: middle domain. Domain boundaries are indicated (amino-acid (aa) numbering). Lower part: complementation analysis of the V.c. ΔclpV secretion defect by plasmid-encoded clpV and its deletion variant lacking the N-terminal domain (ΔN-clpV) using Hcp- and VgrG2-specific antibodies. ClpV levels were monitored by immunoblot analysis using ClpV-specific antibodies. Sup: culture supernatant; total: total cell extract. (B) Purified GST–N-ClpV was coupled to glutathione beads and incubated with soluble cell extracts of V. cholerae V52 ΔclpV. Cell extract incubations with either GST or GST–N-ClpB served as a control. Proteins that were specifically co-purified with GST–N-ClpV (red circles) were identified by mass spectrometry as VCA0107 and VCA0108 and were termed VipA (ClpV-interacting protein A) and VipB, respectively. The positioning of GST, GST–N-ClpV and GST–N-ClpB is indicated. A protein standard is given. (C) Schematic representation of the T6SS encoding gene clusters of V. cholerae, enteroaggregative Escherichia coli (EAEC 42), Edwardsiella tarda and Pseudomonas aeruginosa. VipA, VipB and ClpV encoding genes are coloured in red, yellow and blue, respectively. (D) Purified GST–N-ClpV was coupled to glutathione beads and incubated with purified VipA or VipA/VipB complex. Incubation of VipA or VipA/VipB with empty glutathione beads (−GST–N-ClpV) served as a control. The specific co-sedimentation of VipA/VipB with GST–N-ClpV demonstrates complex formation.