Table 4.
One-Pot Asymmetric Alkyl Addition/Diastereoselective Bromo- and Chlorocyclopropanation from Scheme 7.
| entry | product | % ee (% y) | dra | entry | product | % ee (% y) | dra |
|---|---|---|---|---|---|---|---|
| 1 |
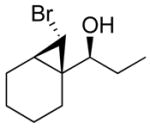 2a
2a
|
99 (70) | > 20:1 | 6 |
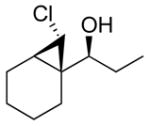 3a
3a
|
99 (65) | > 20:1 |
| 2b |
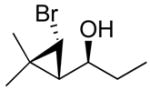 2b
2b
|
95 (75) | > 20:1 | 7b |
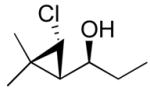 3b
3b
|
95 (70) | > 20:1 |
| 3b |
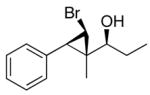 2c
2c
|
96 (80) | > 20:1 | 8 |
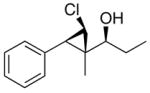 3c
3c
|
96 (70) | > 20:1 |
| 4c |
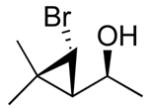 2d
2d
|
99 (70) | > 20:1 | 9c |
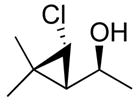 3d
3d
|
99 (59) | > 20:1 |
| 5 |
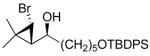 2e
2e
|
97 (77) | > 20:1 | 10 |
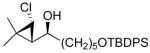 3e
3e
|
97 (70) | > 20:1 |
Determined by 1H NMR analysis of the crude reaction mixture.
Stereochemistry assigned by X-ray analysis, see SI.
10 mol % MIB was used with ZnMe2.
