Figure 6. Relative expression of α-dystroglycan to β-dystroglycan and to total cell protein was reduced in Sphynx cat skeletal muscle.
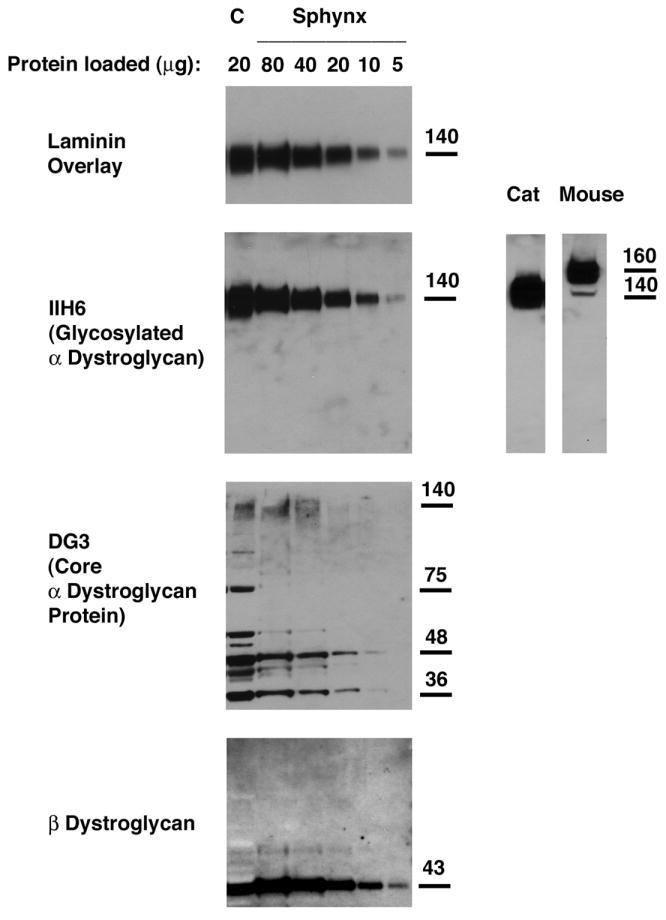
Different amounts of total muscle cell lysates were loaded to compare α- and β-dystroglycan expression by immunoblotting. An antibody that recognizes natively glycosylated α-dystroglycan (IIH6) and one that recognizes α-dystroglycan polypeptide (DG3) were used, and laminin-1 binding to α-dystroglycan was visualized by laminin overlay. Levels of α-dystroglycan, both by IIH6 and DG3 immunoblotting, were lower in Sphynx cat skeletal muscle than in normal controls (C), as was laminin binding to α dystroglycan, when comparing equivalent amounts of loaded protein (20ug). Increasing the amount of Sphynx protein lysate loaded increased IIH6 blotting and laminin binding to levels beyond wild type. α-dystroglycan in normal cat skeletal muscle migrates about 20kDa lower than α-dystroglycan in mouse skeletal muscle.
