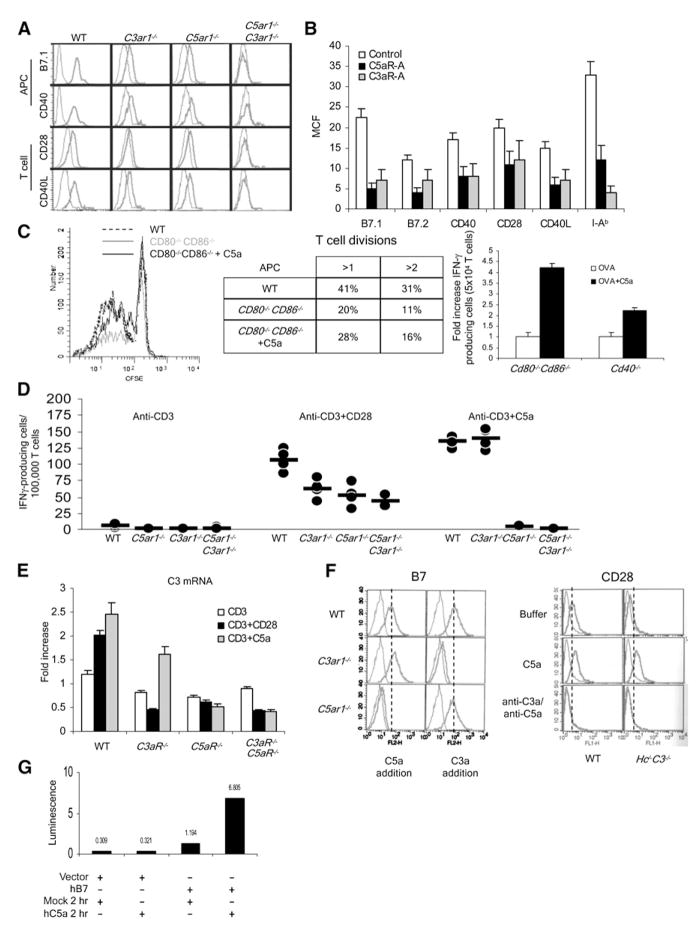
Figure 5. Locally Produced C5a and C3a Influence APC-T Cell Interactions through Regulating Costimulatory-Molecule Expression Levels.
(A) Peritoneal macrophages and CD4+ T cells from naive WT, C3−/−, C3ar1−/−, and C5ar1−/− mice were assayed for B7.1, B7.2, CD40, or CD28, and anti-CD3+anti-CD28-stimulated CD4+ T cells were assayed for CD40L expression levels by flow cytometry. (I-Ab surface expression was also decreased [not shown].)
(B) WT T cells and DCs were incubated for 1 hr at 37°C with 100 ng/ml C5aR-A or C3aR-A, and costimulatory molecule or MHC class II expression was assayed by flow cytometry.
(C) The left side shows that CFSE-labeled Mar T cells were incubated with WT or Cd80−/−Cd86−/− DC and 1 μg/ml Dby peptide ± 100 ng/ml of C5a, and proliferation was assessed by CFSE dilution. In a second experiment (middle), the percentage of cells undergoing greater than one or greater than two divisions was quantified. The right side shows that OT-II T cells were incubated for 18 hr with Cd80−/−Cd86−/− or CD40−/− DCs ± 300 ng/ ml C5a, and IFNγ+ T cells were quantitated by ELISPOT (representative of two experiments; WT versus Cd80−/−Cd86−/−, p < 0.05). C5a was added once at time 0 rather than continuously as occurs with CD28 signaling.
(D) WT, C3ar1−/−, C5ar1−/−, and C5ar1−/−C3ar1−/− T cells were incubated in complete RPMI with anti-CD3 (left), anti-CD3+anti-CD28 (middle), or anti-CD3 + 300 ng/ml C5a (right) and IFNγ+ cells assayed at 48 hr by ELISPOT (n = 5 each group; some dots overlap).
(E) WT, C3ar1−/−, or C5ar1−/− T cells were incubated with buffer, anti-CD3+anti-CD28, or anti-CD3 + 300 ng/ml C5a and complement (C3) mRNA transcripts measured by qPCR (representative of three experiments).
(F) The left side shows that WT, C3ar1−/−, or C5ar1−/− DCs were incubated for 4 hr ± 300 ng/ ml C5a, and Cd80 as well as Cd86 expression was measured by flow cytometry. Similar results were obtained in assays for I-Ab, CD40, with both DCs and macrophages and for CD40L and CD28 with T cells (data not shown). The right side shows that WT or C3−/−Hc−/− T cells were incubated with buffer, C5a, or anti-C3a+anti-C5a mAbs, and CD28 was measured as above.
(G) Base pairs −991 to +72 of the human B7.1 promoter were inserted into a luciferase reporter vector GL4 and transfected into THP-1 cells by electroporation. After overnight incubation in complete medium, the cells were treated with 300 nM of C5a for 2 hr in serum-free media, and luciferase activity was measured with a Lmax Luminometer (Molecular Devices). Each experiment was repeated at least once with similar results. *p < 0.05. All error bars are ± SD.