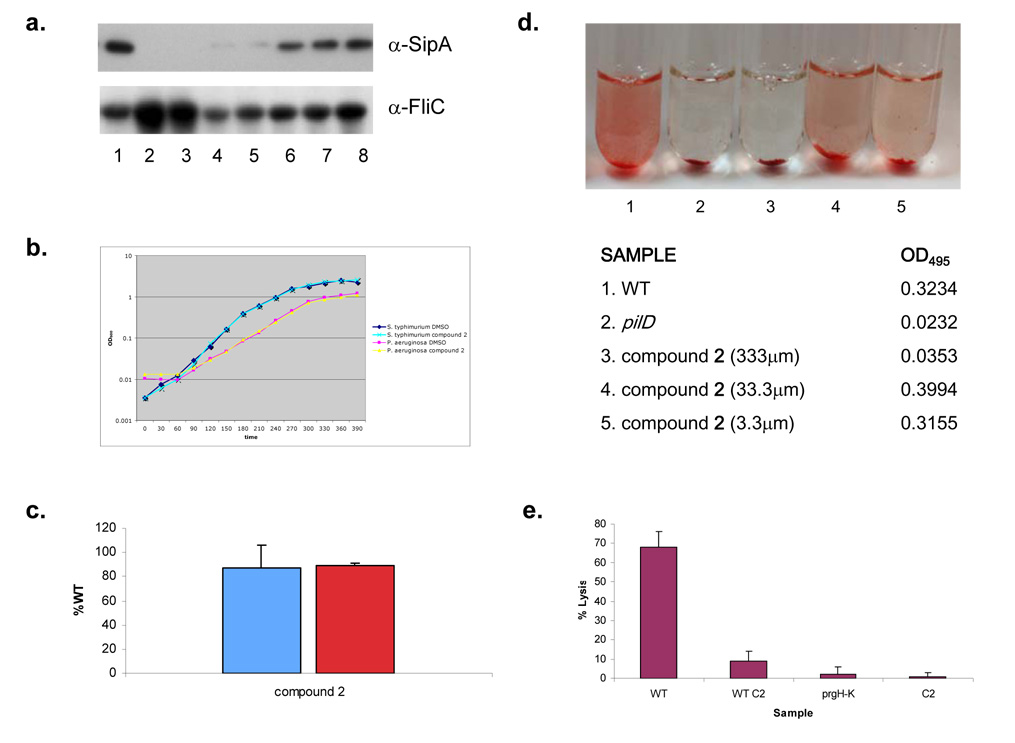
Figure 6. Characterization of secretion inhibition by compound 2.
a, Western Blots of secreted SipA protein as well as the flagellar filament protein, FliC in supernatants from S. typhimurium grown in LB alone or with decreasing concentrations of compound 2. b, Growth curves for S. typhimurium and P. aeruginosa in LB medium with compound 2 or an equal volume of solvent. c, β-galactosidase activity (blue bar) from an iagB-lacZ transcriptional fusion in S. typhimurium or the amount of alkaline phosphotase activity (red bar) observed from a PrgH-PhoA translational fusion in S. typhimurium when grown in the presence of compound 2 (100 µm). Enzymatic activity was recorded as a percent of WT, i.e. the amount of activity observed in the absence of compound. Three independent experiments were performed and the mean standard deviations are shown. d, Elastolytic activity for 18 hour culture supernatants of P. aeruginosa grown in the absence (1) or presence of compound 2 at varying concentrations (3, 4 & 5) was determined using elastin Congo Red as a substrate. Elastase activity was measured as a change in OD495 and can be observed as an increase in the red color of the sample. As a negative control elastase activity was determined for the culture supernatant of a P. aeruginosa T2S mutant (pilD; 2). Pictures and the corresponding OD495 of samples are shown. e, Mouse BMMs were infected with WT S. typhimurium grown in the absence (WT) or presence of 100 µm of compound 2 (WT C2), a T3S mutant (prgH–K) or compound 2 without bacteria (C2). Bacteria were added at an MOI of 40 and cytotoxicity was assessed by LDH release at 60 minutes. Assays were performed in quadruplicate and mean standard deviations are shown.