Abstract
Immune responses to vaccination are tested in clinical trials. This process usually requires years especially when immune memory and persistence are analyzed. Markers able to quickly predict the immune response would be very useful, particularly when dealing with emerging diseases that require a rapid response, such as avian influenza. To address this question we vaccinated healthy adults at days 1, 22, and 202 with plain or MF59-adjuvanted H5N1 subunit vaccines and tested both cell-mediated and antibody responses up to day 382. Only the MF59-H5N1 vaccine induced high titers of neutralizing antibodies, a large pool of memory H5N1-specific B lymphocytes, and H5-CD4+ T cells broadly reactive with drifted H5. The CD4+ response was dominated by IL-2+ IFN-γ− IL-13− T cells. Remarkably, a 3-fold increase in the frequency of virus-specific total CD4+ T cells, measurable after 1 dose, accurately predicted the rise of neutralizing antibodies after booster immunization and their maintenance 6 months later. We suggest that CD4+ T cell priming might be used as an early predictor of the immunogenicity of prepandemic vaccines.
Keywords: H5N1 influenza vaccine, MF59 adjuvant, prepandemic vaccination, immune memory, protection
Influenza pandemics occur with the emergence of new pathogenic strains to which the population is immunologically naive. In recent years the emergence of highly pathogenic H5N1 avian influenza strains, their transmission from poultry to humans, and the increase in global travel have created the potential of a new pandemic and the need to define strategies to limit its spread and mortality (1, 2). Lessons from previous influenza pandemics suggest that case isolation and social distance play a critical role in containing the spread of infection (1, 3). Mathematical models suggest that a vaccine inducing a protective response in 2 weeks, if given at the start of the outbreak, can reduce clinical cases by 90% (1). Correlates of protection to avian influenza have not been established yet and are extrapolated from the experience with seasonal flu, where a serum antibody hemagglutination inhibition (HI) titer of ≥40 is considered protective (4, 5). For avian influenza strains a good correlation was shown between HI titers ≥40 and microneutralization (MN) titers ≥80. This correlation, together with the analysis of sera from patients who recovered from avian influenza infection, supports the use of an MN titer of ≥80 as a correlate of efficacy in avian influenza vaccines (4).
Previous work demonstrated that 2 doses of avian influenza vaccines formulated with a strong adjuvant such as MF59 are required to induce potentially protective titers of neutralizing antibodies broadly reactive to drifted H5 strains (6–10). Those studies also showed that the duration of the antibody response is limited but boosting is effective in subjects that received a successful priming regimen (6–10).
Such considerations support a prime-boost strategy based on 2 immunizations for “prepandemic vaccination” followed by a third “booster dose” at the start of the pandemic outbreak. A drawback to this strategy is the lack of early markers capable of predicting the proportion of the population that develops a memory response after prepandemic vaccination, information currently deduced only post hoc on the basis of the response to the booster dose. To identify an early marker of effective prepandemic priming we analyzed both the antibody and cell-mediated responses in a prime-boost clinical trial. We conducted a phase II study wherein healthy adults received 2 doses of a subunit H5N1 A/Vietnam/1194/2004 vaccine as prepandemic vaccination, followed at 6 months by a third booster dose. The vaccine was either plain (Non-Adj-15) or adjuvanted with MF59 (MF59-H5N1), an oil/water proprietary adjuvant used in seasonal flu vaccines since 1997 (11, 12).
We found that 1 dose of MF59-H5N1 vaccine is sufficient to expand CD4+ T lymphocytes with a Th1-prone effector/memory phenotype; whereas 2 doses are required to expand the pool of H5N1-memory B cells and to elicit high titers of neutralizing antibodies (6–10).
Strikingly, a 3-fold increase in total H5-specific CD4+ T cells after the first dose predicts the rise of MN antibodies to titers ≥80 after booster immunization and their persistence at 6 months with 75 and 85% accuracy, respectively. We suggest that, if confirmed on a larger number of subjects, CD4+ T cell priming can be used as an early measure of vaccine efficacy and can help screen different prepandemic vaccine formulations for their ability to induce immune memory.
Results
Induction of Broadly Reactive H5-CD4+ T Cells.
Forty healthy adults were randomly assigned to 3 groups and immunized with 2 doses of either 15 μg of H5N1 (Non-Adj-15) or MF59-adjuvanted H5N1 at 7.5 or 15 μg of antigen (MF59-H5N1 7.5 and 15). To directly assess priming to H5 we analyzed the T cell response after in vitro stimulation with a library of peptides spanning the whole H5 A/Vietnam/1194/2004 protein (H5-CD4+ T). In parallel we analyzed the T cell response to H5N1, the antigen preparation present in the vaccine (H5N1-CD4+ T). CD4+ T lymphocytes were analyzed by polychromatic flow cytometry to simultaneously measure the frequency of CD3+ CD4+ T lymphocytes and the synthesis of 3 cytokines (IL-2, IFN-γ, and IL-13). This approach allows the enumeration of antigen-specific T cells and a detailed analysis of their functionality (13–16).
We first enumerated total antigen-specific CD4+ T cells by summing the frequency of CD4+ T cells producing all nonoverlapping permutations of the cytokines tested. Low frequencies of H5-CD4+ T cells were detected in the preimmune samples (mean 164 in 106 total CD4+ T cells; Fig. 1A).
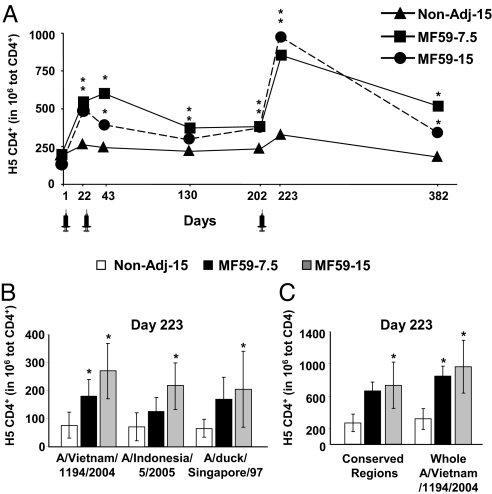
Fig. 1.
One dose of MF59-H5N1 induces the expansion of H5-specific CD4+ T cells, reacting to antigenically distinct H5 proteins. (A) Mean frequency of cytokine+ CD4+ T lymphocytes following in vitro stimulation of PBMC with a library of peptides spanning the whole H5 A/Vietnam/1194/2004. (*, significantly, P < 0.05, different from baseline; Wilcoxon's test for dependent variables). (B and C) Mean frequency (with 95% confidence interval, CI) of cytokine+ CD4+ T lymphocytes after in vitro stimulation of PBMC with a pool of peptides spanning the regions (B) nonconserved or (C) conserved between the indicated H5 strains or the whole H5 A/Vietnam/1194/2004. (*, significant, P < 0.05, differences compared to the Non-Adj-15 group; 1-factor ANOVA with least significant difference post hoc). PBMC were taken at the indicated time point from subjects vaccinated with nonadjuvanted H5N1 at 15 μg/dose (solid triangles or open bars), MF59-adjuvanted H5N1 at 7.5 μg/dose (solid squares or solid bars), and MF59-adjuvanted H5N1 at 15 μg/dose (solid circles or shaded bars).
In subjects that received the plain vaccine (Non-Adj-15) the frequency of H5-CD4+ T lymphocytes increased only 1.4-fold after the first and second dose, did not increase further after booster vaccination, and contracted to values indistinguishable from baseline 6 months following booster immunization (day 382) (Fig. 1A). Remarkably, a single dose of either of the MF59-adjuvanted formulations (day 22) induced a 3-fold increase in the frequency of total H5-CD4+ T lymphocytes; total H5-CD4+ T cells increased only modestly after the second dose (day 43) but remained 2-fold above baseline 3 and 5 months later (days 130 and 202). The booster immunization with MF59-H5N1 expanded the total H5-CD4+ T cells to values 4- and 8-fold above baseline and 2-fold above the frequency observed after the first 2 doses (day 223; Fig. 1A). Of note, 6 months after booster immunization with MF59-H5N1, total frequencies of H5-CD4+ T cells remained above baseline (day 382; Fig. 1A).
We then analyzed the frequency of CD4+ T cells upon in vitro stimulation of peripheral blood mononuclear cells (PBMC) with H5N1 (H5N1-CD4+ T). Few H5N1-CD4+ T cells were detected in the preimmune samples (mean 219 in 106 total CD4). In volunteers that received the plain H5N1 vaccine the frequency of H5N1-CD4+ T cells increased 1.5-fold after the first 2 doses, reached values 3-fold above baseline after booster immunization (day 223), but contracted to baseline frequencies 6 months afterward [day 382; supporting information (SI) Fig. S1]. By contrast, in subjects vaccinated with either adjuvanted formulation, total H5N1-CD4+ T cells increased 2- to 3-fold after the first dose, increased modestly after the second dose, greatly expanded in response to booster immunization (8-fold and 15-fold in the MF59-H5N1 at 7.5 and 15 μg, respectively), and remained 3-fold above baseline 6 months afterward (Fig. S1).
We then tested if total CD4+ T cells, induced by vaccination with clade 1 H5N1 A/Vietnam/1194/2004, reacted with H5 of different clades (http://www.who.int/csr/disease/avian_influenza/guidelines/nomenclature/en/index.html). We compared the T cell response to in vitro stimulation with pools of peptides spanning either conserved or nonconserved regions of H5 A/Vietnam/1194/2004 (clade 1), A/Indonesia/5/05 (clade 2.1), and A/duck/Singapore/97 (clade 0-like). Vaccination with MF59-H5N1, but not with the plain vaccine, expanded H5-CD4+ T cells that responded with similar potency to peptide pools spanning the nonconserved regions of drifted H5 (Fig. 1B). In addition, total CD4+ T cells strongly responded to stimulation with peptides spanning the regions of H5 conserved between the 3 strains tested (Fig. 1C).
In conclusion, the plain H5N1 vaccine expands H5- or H5N1-specific CD4+ T cells only modestly. A single immunization with MF59-H5N1 is sufficient to prime high frequencies of antigen-specific CD4+ T cells that markedly expand in response to booster immunization and recognize H5 of different clades.
H5-CD4+ T Cells Display an Effector/Memory Th1 Phenotype.
We then analyzed the relative proportion of Th1 (IFN-γ+) and Th2 (IL-13+) T cells within H5- and H5N1-CD4+ T lymphocytes (17, 18). The frequency of IL-13+ cells within H5 or H5N1-CD4+ T lymphocytes was extremely low and decreased slightly following vaccination, suggesting that neither the plain nor the MF59-adjuvanted formulation induced a Th2 response (Fig. 2 A and B).
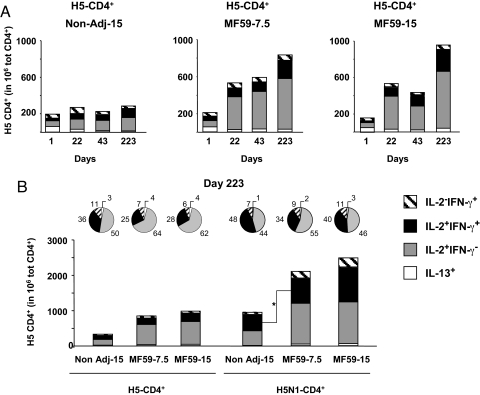
Fig. 2.
Functional characterization of CD4+ T cells induced by vaccination with H5N1 A/Vietnam/1194/2004. Frequency of IL-13+ (open bars), IL2+IFN-γ (shaded bars), IL2+IFN-γ+ (solid bars), and IL2−IFN-γ+ (hatched bars) CD4+ T lymphocytes after a short in vitro stimulation of PBMC with (A and B) a library of peptides spanning H5 A/Vietnam/1194/2004 (H5-CD4+ T) or (B) the H5N1 subunit, the antigen preparation of the vaccine (H5N1-CD4+ T). (B) The pie charts represent the relative contribution (percentage) of each CD4+ T subset to the total response to H5 and H5N1. No significant variation in the proportion of IL-13+/IL-2+IFN-γ−/IL-2+IFN-γ+/IL-2−IFN-γ+ was associated with the plain or the MF59-adjuvanted vaccine formulations (P value >0.1, Kruskal–Wallis H-test, followed by Wilcoxon's test for pairwise comparisons). An asterisk marks the only significant variation observed (Wilcoxon's test, P = 0.049).
We then analyzed at the single-cell level the production of IL-2 and IFN-γ. After immunization with either the plain or the MF59-adjuvanted vaccines, the CD4+ T cell response was dominated by lymphocytes producing IL-2 but not IFN-γ (IL-2+ IFN-γ−), which constituted up to 70% of the total H5- and H5N1-CD4+ T cells (Fig. 2 A and B). Double positive CD4+ (IL-2+ IFN-γ+) T cells were less represented in all vaccinees (average 20% of the H5- and H5N1-CD4+ T cells after the first 2 doses). After booster immunization, H5-CD4+ (IL-2+ IFN-γ+) T cells increased only modestly, whereas double positive H5N1-CD4+ (IL-2+ IFN-γ+) T cells increased to 40% of the responding lymphocytes. Single positive H5- or H5N1-CD4+ (IL-2− IFN-γ+) T cells never contributed more than 5−10% to the total CD4+ response throughout the study (Fig. 2 A and B).
In conclusion, the CD4+ T cell response to H5 peptides and H5N1 protein is dominated by lymphocytes synthesizing IL-2 but not IFN-γ, a CD4+ T cell subpopulation with memory potential but limited effector functionality (14, 19). Mature Th1 effector/memory lymphocytes, synthesizing both IL-2 and IFN-γ, account for 20% of the responding T cells and increase to 40% of H5N1-CD4+ T cells after booster immunization. CD4+ T cells producing IFN-γ, but not IL-2, a more terminally differentiated population with limited memory potential, are less represented (5−10%) (14, 19). A comparative analysis of the proportion of each CD4+ T cell subset to the H5- or H5N1-specific CD4+ T cell response showed no significant variation in response to the plain or MF59-adjuvanted vaccines (P value >0.1).
Expansion of H5N1 Memory B Cells.
Before vaccination, the mean frequency of H5N1-IgG memory B cells (MBC) was ≤1% of total IgG MBC in all groups (Fig. 3). In subjects vaccinated with plain H5N1, only minor changes in the frequency of H5N1-IgG MBC were detected throughout the study (Fig. 3).
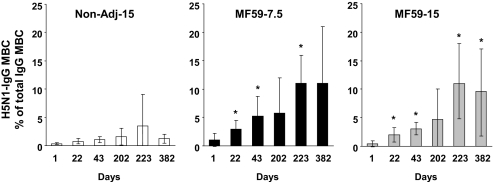
Fig. 3.
Two doses of MF59-H5N1 are required to expand a large and stable pool of H5N1-IgG memory B cells. Mean frequency (with 95% CI) of circulating H5N1-IgG memory B cells (MBC) as percentage of total circulating IgG MBC (*, significant, P < 0.01, different from baseline; Wilcoxon's test for dependent variables).
In contrast, a significant expansion of H5N1-IgG MBC was observed after 2 doses of the MF59-H5N1 vaccines (mean values at day 43 of 5.2 and 3.1% in the MF59-H5N1 at 7.5 and 15 μg, respectively; Fig. 3). In both MF59-adjuvanted groups H5N1-IgG MBC greatly expanded upon booster immunization (mean value at day 223 of 11% in both MF59-H5N1 groups). Six months later (day 382) ≈60% of subjects in both MF59-H5N1 groups maintained frequency of H5N1-IgG MBC 4-fold above baseline (mean values at day 382 of 11 and 9.5% in MF59-H5N1 at 7.5 and 15 μg, respectively; Fig. 3).
In conclusion, 2 doses of MF59-H5N1 vaccine, at either 7.5 or 15 μg, prime a large and stable pool of H5N1-MBC that further expands upon boosting and persists for at least 6 months.
Neutralizing Antibody Responses.
Before vaccination, most subjects had MN titers below the limit of detection. As observed in previous studies (6, 7), a single dose (day 22) did not induce an increase in MN titers, irrespective of the formulation tested (Fig. 4A).
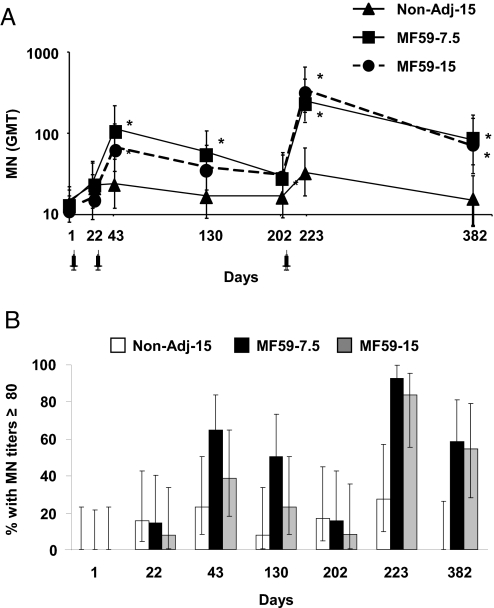
Fig. 4.
Two doses of MF59-H5N1 are required to induce sustained neutralizing antibodies. (A) Geometric mean titers (GMT) with 95% CI of circulating antibodies neutralizing the homologous A/Vietnam/1194/2004 NIBRG-14 recombinant virus in subjects vaccinated with Non-Adj-15 (triangles), MF59–7.5 (squares), and MF59–15 (circles). (*, significant, P < 0.01, different from baseline; Wilcoxon's test for dependent variables). (B) Percentage of subjects displaying MN antibody titers above the potentially protective threshold of 1:80 in Non-Adj-15 (open bars), MF59–7.5 (solid bars), and MF59–15 (shaded bars) groups.
In subjects vaccinated with the plain formulation MN titers rose only slightly above baseline after the second dose and upon booster immunization, but contracted to baseline values 6 months afterward [Fig. 4A; geometric mean ratios above baseline (GMR) were 1.6 at day 43, 2.1 at day 223, and 0.9 at day 382].
Conversely, after 2 doses (day 43) of either adjuvanted vaccine, MN titers rose significantly above baseline (GMR = 8.1 and 5.8 in the MF59–7.5 and MF59–15 groups, respectively) and 64 and 38% of subjects had MN titers ≥80 (Fig. 4B).
Following booster immunization (day 223), in either of the MF59-adjuvanted groups MN titers sharply increased above baseline (GMR = 17 and 34 in the MF59–7.5 and MF59–15 groups, respectively) and 92 and 83% of subjects had MN titers ≥80 (Fig. 4B). Six months after boost (day 382), in both MF59-H5N1 groups MN titers remained >5-fold above baseline and 55% of vaccinees maintained MN titers ≥80 (GMR = 5.6 and 7 in the MF59–7.5 and MF59–15 groups, respectively; Fig. 4).
In conclusion, 2 doses of MF59-adjuvanted H5N1 vaccine are required to induce high, boostable, and sustained MN antibody responses.
Early CD4+ T Cell Expansion Predicts Long-Term MN Response.
Looking for an early predictor of long-term antibody responses, we studied the earliest meaningful time points for H5-CD4+ T cells, MN titers, and H5N1-IgG MBC frequencies.
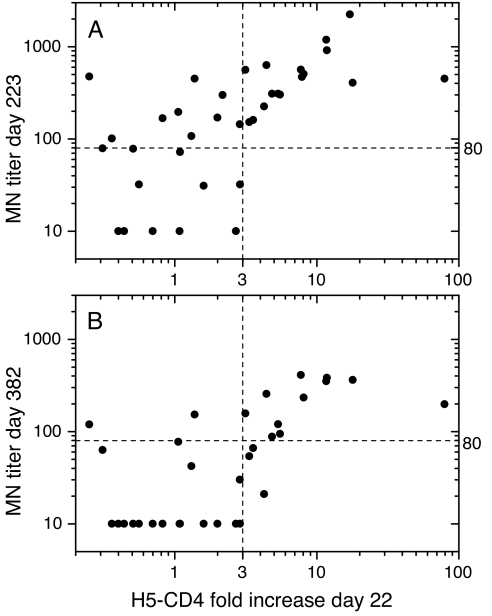
Fig. 5A shows the relationship between the fold increase of total cytokine+ H5-CD4+ T cells, measured at day 22, and MN titers measured at day 223. A rank-correlation analysis of the data indicated a significant correlation between frequency of total H5-CD4+ T cells and MN titers (Spearman's ρ = 0.60, P value <10−4). Furthermore, a ≥3-fold increase in H5-CD4+ T cells was always associated with high MN titers. More specifically, a ≥3-fold expansion of H5-CD4+ T cells at day 22 was significantly associated (Fisher's test, association P value <10−3) with an MN titer ≥80, the proposed threshold of protective antibodies (4), with a predictive accuracy and specificity of 75 and 100%, respectively (Table 1). A similar correlation was found at day 382 (Fig. 5B, Spearman's ρ = 0.54, P value <10−3), with association P value = 10−4 and both predictive accuracy and specificity of 85%.
Fig. 5.
Association between expansion of H5-CD4+ T cells after the first dose and MN titers at later time points. For each subject, the MN titer at day 223 (A) or 382 (B) is plotted vs. the H5-CD4+ T cells fold increase at day 22 over the preimmune time point. Horizontal dashed lines indicate the value of MN titer = 80, the proposed threshold of protective antibodies. Vertical dashed lines indicate the value of H5-CD4+ T cells 3-fold increase.
Table 1.
Expansion of H5-CD4+ T cells ≥3-fold at day 22 predicts MN titer ≥80 at days 223 and 382
| ≥80 | <80 | |||
|---|---|---|---|---|
| MN titer day 223 | ||||
| CD4 fold rise (day 22/baseline) | ≥3 | 16 | 0 | PPV 100% |
| <3 | 9 | 11 | NPV 55% | |
| P value = 0.0005 | Sens. 64% | Spec. 100% | Accuracy 75% | |
| MN titer day 382 | ||||
| CD4 fold rise (day 22/baseline) | ≥3 | 11 | 3 | PPV 79% |
| <3 | 2 | 17 | NPV 90% | |
| P value = 0.0001 | Sens. 85% | Spec. 85% | Accuracy 85% | |
In each 2 × 2 contingency table sensitivity (Sens.), specificity (Spec.), positive predictive value (PPV), negative predictive value (NPV), accuracy, and 2-tailed Fisher's exact association test P-value are shown (see Materials and Methods).
The choice of a 3-fold increase of H5-CD4+ T cells as a predictor for MN ≥80 at days 223 and 382 was supported by the association analysis shown in Fig. S2, where the association P values are shown for all of the range of cutoffs of H5-CD4+ T cells at day 22. Only for a fold increase ≥3 of H5-CD4+ T cells did we observe a highly significant association (Fisher's P value <10−3) with MN titer ≥80 at both days 223 and 382.
A similar analysis showed that MN titers ≥40 at day 43 were associated with MN titers ≥80 at day 223 (Fisher's P value <10−3) and predicted MN response at day 223 with an accuracy of 78% (Fig. S3 and Table S1). However, no MN titer at day 43 was associated with or predicted MN titers ≥80 at day 382 (Fisher's P value >10−2).
Finally, frequency of H5N1-IgG MBC at day 43 was weakly associated with MN titers ≥80 at days 223 and 382 (Fisher's P values = 9 × 10−3 and 2 × 10−3, respectively), with a predictive accuracy of 68% at day 223 and 78% at day 382 (Fig. S4 and Table S2).
A comparison of the various predictors tested (Table S3) shows that total H5-CD4+ T cells 3 weeks after the first dose are the earliest and most accurate predictor of a protective neutralizing antibody response and its persistence.
Discussion
The spread of avian influenza in wild birds and poultry, the identification of cases of direct transmission to humans, and its very high fatality rate have created the specter of a new pandemic. The development of a vaccine capable of eliciting a broad memory immune response, rapidly boostable at the start of the pandemic, is considered the best strategy to provide a first line of defense to the population largely naive to avian influenza. Limitations in developing such a vaccine come from the need to induce a broad memory response in a naive population together with the lack of an early immune marker predictive of vaccination take rate measured as development of immunological memory.
Unlike seasonal influenza, for which vaccine efficacy in humans has been tested both in protection and in challenge studies, for avian influenza there is limited knowledge of the immunological correlates of protection. Comparison of HI and MN assays for seasonal and avian influenza strains showed a good correlation between HI titers of 1:40, considered protective in seasonal influenza, and MN titers of 1:80. Such studies together with results from the analysis of sera from patients who recovered from avian influenza infection support the use of an MN titer of ≥80 as an efficacy endpoint for avian influenza vaccines (4).
To better characterize the immune response to avian flu vaccines and to search for early markers predictive of induction of immune memory, we analyzed the kinetics, magnitude, and quality of the antibody and cell-mediated responses to an avian flu vaccine, plain or adjuvanted with MF59, in a 3 doses prime-boost phase II clinical trial. We found that only the MF59-H5N1 vaccine, containing 7.5 or 15 μg of H5N1 antigen, expands H5N1-CD4+ T cells with a Th1 effector/memory phenotype (IL-2+ IFN-γ−/IL-2+ IFN-γ+), generates a large pool of H5N1-IgG MBC, and generates high and sustained titers of neutralizing antibodies. Moreover, in subjects immunized with MF59-H5N1 both cell-mediated and antibody responses are strongly boosted by a third dose given 5 months after priming, indicative of the induction of immunological memory. Of note, the frequency of H5N1-IgG MBC measured after 3 doses of MF59-adjuvanted vaccines is comparable to that observed after seasonal influenza vaccination (20, 21).
We found that the CD4+ T cell response is measurable after a single immunization with either of the MF59-adjuvanted formulations whereas 2 doses are required to induce a measurable increase in MN antibodies and memory B cells.
A ≥3-fold increase in the frequency of total cytokine+ H5-CD4+ T cells after the first dose (day 22) predicts the rise of MN titers ≥80 after booster vaccination and their maintenance 6 months later with 75 and 85% accuracy, respectively.
The other parameters studied also showed some correlation but it was never as good as the one observed with total cytokine+ H5-CD4+ T cells. For instance, after the second dose, at day 43, MN titers ≥40 were predictive of MN titers ≥80 at 3 weeks after boost, but not of their maintenance 6 months later. Similarly, at day 43, frequency of memory B cells ≥2.5% predicted the rise and persistence of MN titers ≥80 after booster immunization, although less accurately than H5-CD4+ T cells at day 22.
We therefore conclude that a ≥3-fold increase in the frequency of total H5-CD4+ T cells after the first dose was the earliest and most accurate predictor of the rise of MN antibodies to potentially protective titers after booster vaccination and of their maintenance 6 months later.
Given that CD4+ T cells are not directly responsible for antibody production and that their expansion is the earliest measurable immunological parameter predictive of the neutralizing antibody response, we suggest that, if confirmed on a larger subject database, CD4+ T cell responses after the first immunization could be used as an early measure of prepandemic vaccination take rate and become a valuable tool for comparing the effectiveness of different vaccine formulations.
The predictive association of CD4+ T cell responses and antibody responses is not surprising because CD4+ help is required for the optimal activation and early clonal expansion of B cells, for the initiation and maintenance of germinal center reaction, and, ultimately, for the generation of long-lived plasma and memory B cells (22–26). Indeed the groups that had a significant increase in the total CD4+ T cell response after the first dose had significant increase in memory B cells after the second and the third dose (Fig. 3). In addition to their helper function, several preclinical studies suggest that influenza-specific CD4+ T cells can accelerate recovery via a direct effector function (27–29).
We also assessed the cytokine profile of antigen-specific CD4+ T cells elicited by vaccination. To this aim we analyzed at a single-cell level synthesis of IL-2, IFN-γ, and IL-13 as prototypic of Th1 (IL-2 and IFN-γ) and Th2 (IL-2 and IL-13) polarized responses (17, 18). The ability of antigen-specific CD4+ T cells to produce IL-2 was also used as a predictor of the ability of CD4+ T cells to survive in vivo and proliferate upon challenge (13, 14). We choose IL-13 over IL-4 because it has been reported that all CD4+ T cells that produce IL-4 also produce IL-13 and that production of IL-13 is more sustained over time (15). In addition, the effects of IL-4 and IL-13 on human B cells are largely similar because IL-13 does enhance production of IgM, IgG, and IgA (16, 30). Such analysis showed that the frequency of H5-specific CD4+ T cells synthesizing IL-13 is extremely low and decreases upon vaccination, suggesting that neither the plain nor the MF59-adjuvanted formulation induces a Th2 response.
This analysis also showed that the antigen-specific CD4+ T cell response is dominated by lymphocytes synthesizing IL-2 but not IFN-γ, a CD4+ T cell subpopulation with memory potential but limited effector functionality (14, 19) followed by mature Th1 effector/memory lymphocytes (synthesizing both IL-2 and IFN-γ). CD4+ T cells producing IFN-γ, but not IL-2, a more terminally differentiated population with limited memory potential, were less represented in all groups (14, 19).
In conclusion, the CD4 response we detected after vaccination is dominated by IL-2+ IFN-γ− T cells with only 20−40% of lymphocytes synthesizing both cytokines, indicating that limiting the analysis to IFN-γ does not allow a full measure of the elicited T cell response and that CD4+ T cells elicited by prepandemic vaccination retain memory potential. It is also of interest that the difference in the CD4+ T cell response between subjects vaccinated with the plain vs. the MF59-adjuvanted vaccines was limited to the frequency of the responding CD4+ T cells with no significant impact on their cytokine profile, suggesting that formulation with MF59 affects the magnitude but not the quality of the elicited T cell response. These findings in humans are in contrast with studies in BALB/c mice where MF59 has been reported to induce a Th2 response (12), thus emphasizing the need of testing several mouse strains to characterize adjuvant activity.
Finally, the finding that CD4+ T cells primed by vaccination with clade 1 H5N1 (A/Vietnam/1194/2004) react with H5 proteins of different clades (clade 0-like and clade 2) supports the use of MF59-adjuvanted prepandemic vaccines to induce broadly reactive CD4+ T cells that could exert their helper and effector functions toward antigenically distinct H5 strains.
Although the implementation of prepandemic vaccination will require complex cost–benefit analyses, the data presented collectively support the use of MF59-adjuvanted vaccines to prime the population against a variety of avian influenza viruses.
Materials and Methods
Study Protocol.
This was a phase-II, randomized, controlled, observer-blind, single-center study, carried out in Italy from 2006 to 2008. The study protocol (registered at Clinical Trials.gov as NCT 00382187) was in accordance with the Helsinki Declaration and Good Clinical Practice principles and approved by the local Ethics Committee. After giving their informed consent, 40 adults were enrolled in the study: 13 subjects received 15 μg of plain H5N1 (Non-Adj-15), 14 subjects received 7.5 μg of H5N1 adjuvanted with MF59 (MF59–7.5, Aflunov), and 13 subjects received 15 μg of H5N1 adjuvanted with MF59 (MF59–15). The vaccine, a monovalent H5N1 subunit from the A/Vietnam/1194/2004 influenza virus obtained by reverse genetics (NIBRG-14) and grown in hens' eggs, was administered at days 1, 22, and 202 in the deltoid muscle. See also SI Text.
Peripheral Blood Mononuclear Cells Preparation.
PBMC were isolated by standard Ficoll gradient (Amersham Pharmacia) centrifugation, frozen, and thawed as detailed in SI Text.
Analysis of Antigen-Specific T Cell Response.
The antigen-specific T cell response was assessed by stimulating PBMC with the different peptide pools (final concentration of each individual peptide: 2.5 μg/ml) or H5N1 (final concentration: 1 μg/ml), fixed and stained for analysis with polychromatic flow cytometry as detailed in SI Text.
Enumeration of H5N1-Specific MBC.
Frequencies of MBC were determined by the ELISA-coupled limiting dilution assay described elsewhere (26) as detailed in SI Text.
Titration of Neutralizing Antibodies.
Titers of H5N1-specific antibodies were determined in heat-inactivated pre- and postvaccination sera as described (6) and detailed in SI. Titers are expressed as reciprocal value of the highest dilution giving ≥50% neutralization of virus growth.
Descriptive Statistics.
Descriptive statistics were calculated by vaccine group, using the Statistical Analysis System (SAS) software version 9.1 (SAS Institute) as described in detail in SI Text.
Tests of Correlation, Association, and Predictivity.
An ad hoc perl program that utilizes the fisher.test() routine from the R package version 2.4.0 (31) was used to evaluate the association, within the whole data set, between the fold increase in H5-CD4+ T cells at days 22, 43, 120, 202, and 223 over day 1 and MN titers at days 43, 223, and 382 (Fig. 5 and Fig. S2) and the frequency of H5N1-IgG MBC or MN titers at day 43 and MN titers at days 223 and 382 (Figs. S3 and S4).
For each predictor candidate (H5-CD4+ T cells, H5N1-IgG MBC, or MN titers) different cutoff values were tested for association with the efficacy endpoint (MN titer ≥80) by calculating the respective 2-tailed P-values of Fisher's exact test. The association was considered significant for P < 0.01 and highly significant for P < 0.001.
On the basis of the results from the association test, we selected the best cutoff value for each potential predictor: expansion of H5-CD4+ T cells ≥3-fold above day 1, H5N1-IgG MBC ≥2.5% of total MBC, and MN titer ≥40 at day 43. For each pair predictor/endpoint a 2 × 2 contingency table was populated with the number of true positives (TP), false positives (FP), false negatives (FN), and true negatives (TN). For each contingency table the following quantities were calculated: accuracy = (TP + TN)/N, negative predictive value = TN/(TN + FN), positive predictive value = TP/(TP + FP), specificity = TN/(FP + TN), and sensitivity = TP/(TP + FN), where N is the sample size.
When considering all of the 6 predictors jointly as in Table S3 (H5-CD4+ T cells, H5N1-IgG MBC, and MN titers at days 22 and 43), a multitest Bonferroni correction for the association P value was applied [p.adjust() routine in R package version 2.4.0 (ref. 31)].
Supplementary Material
Acknowledgments.
We thank Michaela Praus, Simona Toti, and Sergio Abrignani for helpful discussion and M. Giotti, E. Montomoli, C. Gentile, the personnel of the AUSL Azienda Unità Sanitaria Locale 7 in Siena, and all of the volunteers for their help.
Footnotes
Conflict of interest statement: G.G., D.M., L.Z., M.B., S.N., S.T., C.S., A.K.H., V.B., A.B., R.R., G.D.G., and F.C. are employees of Novartis Vaccines, the sponsor of the study; and E.B. and C.M. have a PhD grant from Novartis Vaccines.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813390106/DCSupplemental.
References
- 1.Ferguson NM, et al. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdel-Ghafar AN, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358(3):261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson I, Nicholson KG, Wood JM, Zambon MC, Katz JM. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect Dis. 2004;4(8):499–509. doi: 10.1016/S1473-3099(04)01105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichelberger M, et al. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10–11, 2007. Vaccine. 2008;26(34):4299–4303. doi: 10.1016/j.vaccine.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 5.de Jong JCPA, Beyer WEP, Rimmelzwaan GF, Boon ACM, Osterhaus ADME. Haemagglutination-inhibiting antibody to influenza virus. In: Brown FHL, Schild GC, editors. Laboratory Correlates of Immunity to Influenza Reassessment. Vol 115. Basel: Karger; 2003. pp. 63–73. [Google Scholar]
- 6.Nicholson KG, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357(9272):1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 7.Atmar RL, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006;43(9):1135–1142. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 8.Leroux-Roels I, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370(9587):580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson I, et al. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J Infect Dis. 2005;191(8):1210–1215. doi: 10.1086/428948. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson I, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003;21(15):1687–1693. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- 11.Ott G, et al. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 12.Podda A, Del Giudice G. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines. 2003;2(2):197–203. doi: 10.1586/14760584.2.2.197. [DOI] [PubMed] [Google Scholar]
- 13.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 14.Darrah PA, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13(7):843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 15.Jung T, Wijdenes J, Neumann C, de Vries JE, Yssel H. Interleukin-13 is produced by activated human CD45RA+ and CD45RO+ T cells: modulation by interleukin-4 and interleukin-12. Eur J Immunol. 1996;26(3):571–577. doi: 10.1002/eji.1830260311. [DOI] [PubMed] [Google Scholar]
- 16.Aversa G, et al. An interleukin 4 (IL-4) mutant protein inhibits both IL-4 or IL-13-induced human immunoglobulin G4 (IgG4) and IgE synthesis and B cell proliferation: support for a common component shared by IL-4 and IL-13 receptors. J Exp Med. 1993;178(6):2213–2218. doi: 10.1084/jem.178.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 19.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki S, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81(1):215–228. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrammert J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 23.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27(2):190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 25.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 26.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298(5601):2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 27.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7(5):449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 28.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177(5):2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 29.Swain SL, et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 31.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.